Showing Spotlights 1441 - 1448 of 2786 in category All (newest first):
 Buildings and other man-made structures consume as much as 30-40% of the primary energy in the world, mainly for heating, cooling, ventilation, and lighting. 'Smart' windows are expected to play a significant role in reducing the energy consumption of homes in two ways: by generating energy themselves; and by providing better insulation by allowing light in and keeping the heat out (in hot summers) or in (in cold winters). Vanadium dioxide (VO2) has long been recognized as a a material of significant technological interest for optics and electronics and a promising candidate for making 'smart' windows: it can transition from a transparent semiconductive state at low temperatures, allowing infrared radiation through, to an opaque metallic state at high temperatures, while still allowing visible light to get through. In new work, researchers have now offered a simple method for promoting the production of monoclinic VO2 nanoparticles by doping.
Buildings and other man-made structures consume as much as 30-40% of the primary energy in the world, mainly for heating, cooling, ventilation, and lighting. 'Smart' windows are expected to play a significant role in reducing the energy consumption of homes in two ways: by generating energy themselves; and by providing better insulation by allowing light in and keeping the heat out (in hot summers) or in (in cold winters). Vanadium dioxide (VO2) has long been recognized as a a material of significant technological interest for optics and electronics and a promising candidate for making 'smart' windows: it can transition from a transparent semiconductive state at low temperatures, allowing infrared radiation through, to an opaque metallic state at high temperatures, while still allowing visible light to get through. In new work, researchers have now offered a simple method for promoting the production of monoclinic VO2 nanoparticles by doping.
Aug 27th, 2012
 Micro- and nanoporous materials can widely be found in nature, be it zeolite minerals, cell membranes, or diatom skeletons. Researchers are developing artificial analogues of such materials, i.e. nanoporous materials, for industrial applications in areas such as catalysis, water purification, environmental clean-up, molecular separation and proton exchange membranes for fuel cells. Manufacturing nanosieves with straight nanopores is still challenging, especially when the pore size is less than 10 nm. Researchers in Korea have now developed a novel material and fabrication technique that allows easy fabrication of nanosieves with sub-10 nm nanopores with straight pore-structure. With it, controlling the pore size from sub-nm to 5 nm becomes very easy.
Micro- and nanoporous materials can widely be found in nature, be it zeolite minerals, cell membranes, or diatom skeletons. Researchers are developing artificial analogues of such materials, i.e. nanoporous materials, for industrial applications in areas such as catalysis, water purification, environmental clean-up, molecular separation and proton exchange membranes for fuel cells. Manufacturing nanosieves with straight nanopores is still challenging, especially when the pore size is less than 10 nm. Researchers in Korea have now developed a novel material and fabrication technique that allows easy fabrication of nanosieves with sub-10 nm nanopores with straight pore-structure. With it, controlling the pore size from sub-nm to 5 nm becomes very easy.
Aug 24th, 2012
 Man-made micro- and nanoscale motors have received a tremendous recent interest owing to their great potential for diverse potential applications, ranging from targeted drug delivery, microchip diagnostics or environmental remediation. Particular attention has been given to self-propelled chemically-powered micro/nanoscale motors, such as catalytic nanowires, microtube engines, or spherical Janus microparticles. Although significant progress over the past 10 years has greatly advanced the capabilities of these tiny man-made machines, such catalytic motors have predominantly relied on an external hydrogen peroxide fuel that impedes many practical applications. Researchers have now demonstrated the first example of a water-driven bubble-propelled micromotor that eliminates the requirement for the common hydrogen peroxide fuel.
Man-made micro- and nanoscale motors have received a tremendous recent interest owing to their great potential for diverse potential applications, ranging from targeted drug delivery, microchip diagnostics or environmental remediation. Particular attention has been given to self-propelled chemically-powered micro/nanoscale motors, such as catalytic nanowires, microtube engines, or spherical Janus microparticles. Although significant progress over the past 10 years has greatly advanced the capabilities of these tiny man-made machines, such catalytic motors have predominantly relied on an external hydrogen peroxide fuel that impedes many practical applications. Researchers have now demonstrated the first example of a water-driven bubble-propelled micromotor that eliminates the requirement for the common hydrogen peroxide fuel.
Aug 23rd, 2012
 Chemical and analytical processes using channel-based microfluidics have many advantages, such as the reduced use of chemical reagents and solvents; precisely controlled reaction condition; much shortened reaction time; and the ability to integrate into a digital device. However, it is difficult with channel microfluidics to handle just a single droplet. In contrast with the microchannel-based fluidics, the manipulation of discrete droplets without using channels is a new field. Here, a liquid droplet is not confined to a closed channel and there is no risk of it being adsorbed on a channel wall. A 'liquid marble' coated with micro- or nanoparticles is a novel kind of microfluidic device, one that is especially useful for handling single liquid droplet. Researchers have now come up with the idea of developing 'liquid metal marbles' when they wanted to develop a flexible conductive system for electronic and electromagnetic units.
Chemical and analytical processes using channel-based microfluidics have many advantages, such as the reduced use of chemical reagents and solvents; precisely controlled reaction condition; much shortened reaction time; and the ability to integrate into a digital device. However, it is difficult with channel microfluidics to handle just a single droplet. In contrast with the microchannel-based fluidics, the manipulation of discrete droplets without using channels is a new field. Here, a liquid droplet is not confined to a closed channel and there is no risk of it being adsorbed on a channel wall. A 'liquid marble' coated with micro- or nanoparticles is a novel kind of microfluidic device, one that is especially useful for handling single liquid droplet. Researchers have now come up with the idea of developing 'liquid metal marbles' when they wanted to develop a flexible conductive system for electronic and electromagnetic units.
Aug 20th, 2012
 Thanks to nanotechnologies, in particular nanoelectronics, the medical sector is about to undergo deep changes by exploiting the traditional strengths of the semiconductor industry - miniaturization and integration. While conventional electronics have already found many applications in biomedicine - medical monitoring of vital signals, biophysical studies of excitable tissues, implantable electrodes for brain stimulation, pacemakers, and limb stimulation - the use of nanomaterials and nanoscale applications will bring a further push towards implanted electronics in the human body. A new perspective article provides an overview of nanoelectronics' potential in the biomedical sciences.
Thanks to nanotechnologies, in particular nanoelectronics, the medical sector is about to undergo deep changes by exploiting the traditional strengths of the semiconductor industry - miniaturization and integration. While conventional electronics have already found many applications in biomedicine - medical monitoring of vital signals, biophysical studies of excitable tissues, implantable electrodes for brain stimulation, pacemakers, and limb stimulation - the use of nanomaterials and nanoscale applications will bring a further push towards implanted electronics in the human body. A new perspective article provides an overview of nanoelectronics' potential in the biomedical sciences.
Aug 16th, 2012
 Recent advances in materials, fabrication strategies and device designs for flexible and stretchable electronics and sensors make it possible to envision a not-too-distant future where ultra-thin, flexible circuits based on inorganic semiconductors can be wrapped and attached to any imaginable surface, including body parts and even internal organs. Robotic technologies will also benefit as it becomes possible to fabricate 'electronic skin' that, for instance, could allow surgical robots to interact, in a soft contacting mode, with their surroundings through touch. Researchers have now demonstrated that they can integrate high-quality silicon and other semiconductor devices on thin, stretchable sheets, to make systems that not only match the mechanics of the epidermis, but which take the full three dimensional shapes of the fingertip - and, by extension, other appendages or even internal organs, such as the heart.
Recent advances in materials, fabrication strategies and device designs for flexible and stretchable electronics and sensors make it possible to envision a not-too-distant future where ultra-thin, flexible circuits based on inorganic semiconductors can be wrapped and attached to any imaginable surface, including body parts and even internal organs. Robotic technologies will also benefit as it becomes possible to fabricate 'electronic skin' that, for instance, could allow surgical robots to interact, in a soft contacting mode, with their surroundings through touch. Researchers have now demonstrated that they can integrate high-quality silicon and other semiconductor devices on thin, stretchable sheets, to make systems that not only match the mechanics of the epidermis, but which take the full three dimensional shapes of the fingertip - and, by extension, other appendages or even internal organs, such as the heart.
Aug 15th, 2012
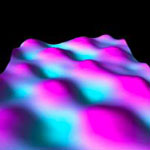 Doping, the process of adding impurity atoms to semiconductors to provide free carriers for conduction, has been pivotal to microelectronics since its early stages. In particular doping germanium at high concentrations to make it highly conductive is the subject of intense research, because it lies at the heart of novel developments in integrated silicon-compatible lasers and quantum information processing devices. Researchers have now demonstrated a method to densely pack dopant molecules on the germanium surface, which then self-organize to form molecular patterns with one phosphorus dopant atom every two germanium atoms. The key finding is that when you deposit phosphine molecules on a germanium surface, they naturally form molecular patterns with one phosphorus atom every two germanium atoms that densely pack the surface.
Doping, the process of adding impurity atoms to semiconductors to provide free carriers for conduction, has been pivotal to microelectronics since its early stages. In particular doping germanium at high concentrations to make it highly conductive is the subject of intense research, because it lies at the heart of novel developments in integrated silicon-compatible lasers and quantum information processing devices. Researchers have now demonstrated a method to densely pack dopant molecules on the germanium surface, which then self-organize to form molecular patterns with one phosphorus dopant atom every two germanium atoms. The key finding is that when you deposit phosphine molecules on a germanium surface, they naturally form molecular patterns with one phosphorus atom every two germanium atoms that densely pack the surface.
Aug 14th, 2012
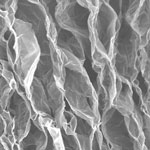 Integration of graphene sheets and its functional derivatives into three-dimensional macroscopic structures is drawing much attention since it is an essential step to explore the advanced properties of individual graphene sheets for practical applications, such as chemical filters and electrodes for energy storage devices. However, a major problem in scaling up production of graphene is the tendency of individual graphene sheets to aggregate due to strong van der Waals attraction. Restacking of sheets not only reduces their solution processability, but also compromises their properties such as accessible surface area. A novel approach uses a simple leavening strategy to prepare reduced graphene oxide (rGO) foams with porous and continuous cross-linked structures from freestanding compact graphene oxide layered films. The whole process is more like making graphene "bread". The rGO foams perform excellently as flexible electrode materials for supercapacitors and selective organic absorbents.
Integration of graphene sheets and its functional derivatives into three-dimensional macroscopic structures is drawing much attention since it is an essential step to explore the advanced properties of individual graphene sheets for practical applications, such as chemical filters and electrodes for energy storage devices. However, a major problem in scaling up production of graphene is the tendency of individual graphene sheets to aggregate due to strong van der Waals attraction. Restacking of sheets not only reduces their solution processability, but also compromises their properties such as accessible surface area. A novel approach uses a simple leavening strategy to prepare reduced graphene oxide (rGO) foams with porous and continuous cross-linked structures from freestanding compact graphene oxide layered films. The whole process is more like making graphene "bread". The rGO foams perform excellently as flexible electrode materials for supercapacitors and selective organic absorbents.
Aug 9th, 2012
 Buildings and other man-made structures consume as much as 30-40% of the primary energy in the world, mainly for heating, cooling, ventilation, and lighting. 'Smart' windows are expected to play a significant role in reducing the energy consumption of homes in two ways: by generating energy themselves; and by providing better insulation by allowing light in and keeping the heat out (in hot summers) or in (in cold winters). Vanadium dioxide (VO2) has long been recognized as a a material of significant technological interest for optics and electronics and a promising candidate for making 'smart' windows: it can transition from a transparent semiconductive state at low temperatures, allowing infrared radiation through, to an opaque metallic state at high temperatures, while still allowing visible light to get through. In new work, researchers have now offered a simple method for promoting the production of monoclinic VO2 nanoparticles by doping.
Buildings and other man-made structures consume as much as 30-40% of the primary energy in the world, mainly for heating, cooling, ventilation, and lighting. 'Smart' windows are expected to play a significant role in reducing the energy consumption of homes in two ways: by generating energy themselves; and by providing better insulation by allowing light in and keeping the heat out (in hot summers) or in (in cold winters). Vanadium dioxide (VO2) has long been recognized as a a material of significant technological interest for optics and electronics and a promising candidate for making 'smart' windows: it can transition from a transparent semiconductive state at low temperatures, allowing infrared radiation through, to an opaque metallic state at high temperatures, while still allowing visible light to get through. In new work, researchers have now offered a simple method for promoting the production of monoclinic VO2 nanoparticles by doping. 
 Subscribe to our Nanotechnology Spotlight feed
Subscribe to our Nanotechnology Spotlight feed





