Showing Spotlights 2497 - 2504 of 2838 in category All (newest first):
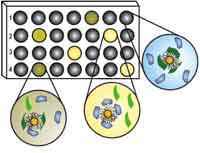 Biomarkers are of increasing importance in modern medicine for the purpose of early detection and diagnosis of a disease, for instance cancer. Biomarkers are mostly protein molecules that can be measured in blood, other body fluids, and tissues to assess the presence or state of a disease. For example, the presence of an antibody may indicate an infection or an antigen, such as PSA, might indicate the presence of prostate-specific cancer cells. Although protein-based approaches to early detection and diagnosis of cancer have a clear advantage over other, more invasive, methods, protein detection is a challenging problem owing to the structural diversity and complexity of the target analytes. State-of-the-art detection methods have limited application due to their high production cost and instability. Another limitation of current proteomic diagnostics is the limitation of arrays to one or a few markers only; in other words, you can only test for the specific markers that you are looking for and not generally measure levels of proteins in your blood in order to detect anomalies. A novel nanotechnology based protein detector array could change that.
Biomarkers are of increasing importance in modern medicine for the purpose of early detection and diagnosis of a disease, for instance cancer. Biomarkers are mostly protein molecules that can be measured in blood, other body fluids, and tissues to assess the presence or state of a disease. For example, the presence of an antibody may indicate an infection or an antigen, such as PSA, might indicate the presence of prostate-specific cancer cells. Although protein-based approaches to early detection and diagnosis of cancer have a clear advantage over other, more invasive, methods, protein detection is a challenging problem owing to the structural diversity and complexity of the target analytes. State-of-the-art detection methods have limited application due to their high production cost and instability. Another limitation of current proteomic diagnostics is the limitation of arrays to one or a few markers only; in other words, you can only test for the specific markers that you are looking for and not generally measure levels of proteins in your blood in order to detect anomalies. A novel nanotechnology based protein detector array could change that.
Jul 2nd, 2007
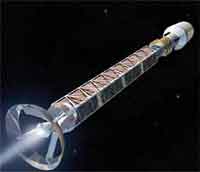 Nanotechnology will play an important role in future space missions. Nanosensors, dramatically improved high-performance materials, or highly efficient propulsion systems are but a few examples. In previous Nanowerk Spotlights we reported about nanotechnology propulsion technology, such as nano field emission thrusters, and the use of carbon nanotubes to harden electronic components in space. This last aspect, radiation shielding, is also where nanotechnology could make a major contribution to human space flight. NASA says that the risks of exposure to space radiation are the most significant factor limiting humans�?? ability to participate in long-duration space missions. A lot of research therefore focuses on developing countermeasures to protect astronauts from those risks. To meet the needs for radiation protection as well as other requirements such as low weight and structural stability, spacecraft designers are looking for materials that help them develop multifunctional spacecraft hulls. Advanced nanomaterials such as the newly developed, isotopically enriched boron nanotubes could pave the path to future spacecraft with nanosensor-integrated hulls that provide effective radiation shielding as well as energy storage.
Nanotechnology will play an important role in future space missions. Nanosensors, dramatically improved high-performance materials, or highly efficient propulsion systems are but a few examples. In previous Nanowerk Spotlights we reported about nanotechnology propulsion technology, such as nano field emission thrusters, and the use of carbon nanotubes to harden electronic components in space. This last aspect, radiation shielding, is also where nanotechnology could make a major contribution to human space flight. NASA says that the risks of exposure to space radiation are the most significant factor limiting humans�?? ability to participate in long-duration space missions. A lot of research therefore focuses on developing countermeasures to protect astronauts from those risks. To meet the needs for radiation protection as well as other requirements such as low weight and structural stability, spacecraft designers are looking for materials that help them develop multifunctional spacecraft hulls. Advanced nanomaterials such as the newly developed, isotopically enriched boron nanotubes could pave the path to future spacecraft with nanosensor-integrated hulls that provide effective radiation shielding as well as energy storage.
Jun 29th, 2007
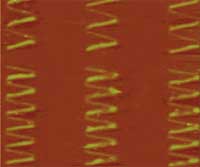 Nanoimprinting lithography (NIL) is a simple pattern transfer process that is emerging as an alternative nanopatterning technology to traditional photolithography. NIL allows the fabrication of two-dimensional or three-dimensional structures with submicrometer resolution and the patterning and modification of functional materials. A key benefit of nanoimprint lithography is its sheer simplicity. There is no need for complex optics or high-energy radiation sources with a nanoimprint tool. There is no need for finely tailored photoresists designed for both resolution and sensitivity at a given wavelength. The simplified requirements of the technology allow low-cost, high-throughput production processes of various nanostructures with operational ease. NIL already has been applied in various fields such as biological nanodevices, nanophotonic devices, organic electronics, and the patterning of magnetic materials. Researchers at Berkeley have taken this process one step further by demonstrating that direct nanoimprinting of metal nanoparticles enables low temperature metal deposition as well as high-resolution patterning. This approach has substantial potential to take advantage of nanoimprinting for the application in ultralow cost, large area printed electronics.
Nanoimprinting lithography (NIL) is a simple pattern transfer process that is emerging as an alternative nanopatterning technology to traditional photolithography. NIL allows the fabrication of two-dimensional or three-dimensional structures with submicrometer resolution and the patterning and modification of functional materials. A key benefit of nanoimprint lithography is its sheer simplicity. There is no need for complex optics or high-energy radiation sources with a nanoimprint tool. There is no need for finely tailored photoresists designed for both resolution and sensitivity at a given wavelength. The simplified requirements of the technology allow low-cost, high-throughput production processes of various nanostructures with operational ease. NIL already has been applied in various fields such as biological nanodevices, nanophotonic devices, organic electronics, and the patterning of magnetic materials. Researchers at Berkeley have taken this process one step further by demonstrating that direct nanoimprinting of metal nanoparticles enables low temperature metal deposition as well as high-resolution patterning. This approach has substantial potential to take advantage of nanoimprinting for the application in ultralow cost, large area printed electronics.
Jun 27th, 2007
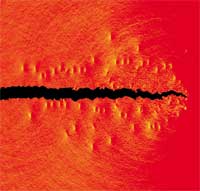 Bone is one of the most fascinating materials that has evolved in nature. There are 206 bones in your body - did you know that a newborn has 350 bones but they fuse together as you grow? - more than half of them in your hands and feet. These bones not only protect your organs, support your body against gravity's pull and allow you to move but they also are living tissues that produce blood cells and store important minerals. Not only important for humans, bones are the essential part of the endoskeleton of all vertebrates. Bone is a composite material of the mineral calcium hydroxyapatite and tropocollagen molecules (the fragile and soluble form of collagen when first synthesized by fibroblasts). It forms an extremely tough, yet lightweight material and its properties and behavior are of great interest to scientists and materials engineers. For instance, very little is known about the fracture behavior of bone and all such studies have been conducted at scales much larger than the nanoscale that explicitly considers individual tropocollagen molecules and mineral particles. New research at MIT has discovered a previously unknown toughening mechanism of bone that operates at the nanoscale - the level of individual collagen molecules and nano-platelets of hydroxyapatite. This breakthrough work lays the foundation for new materials design that includes the nanostructure as a specific 'design variable' and may help engineers to design materials from the bottom up by including hierarchies as a design parameter.
Bone is one of the most fascinating materials that has evolved in nature. There are 206 bones in your body - did you know that a newborn has 350 bones but they fuse together as you grow? - more than half of them in your hands and feet. These bones not only protect your organs, support your body against gravity's pull and allow you to move but they also are living tissues that produce blood cells and store important minerals. Not only important for humans, bones are the essential part of the endoskeleton of all vertebrates. Bone is a composite material of the mineral calcium hydroxyapatite and tropocollagen molecules (the fragile and soluble form of collagen when first synthesized by fibroblasts). It forms an extremely tough, yet lightweight material and its properties and behavior are of great interest to scientists and materials engineers. For instance, very little is known about the fracture behavior of bone and all such studies have been conducted at scales much larger than the nanoscale that explicitly considers individual tropocollagen molecules and mineral particles. New research at MIT has discovered a previously unknown toughening mechanism of bone that operates at the nanoscale - the level of individual collagen molecules and nano-platelets of hydroxyapatite. This breakthrough work lays the foundation for new materials design that includes the nanostructure as a specific 'design variable' and may help engineers to design materials from the bottom up by including hierarchies as a design parameter.
Jun 26th, 2007
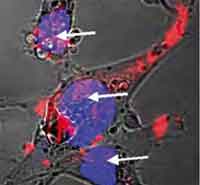 In chemotherapy doctors are using a carpet bombing approach to destroy cancer cells: the patient is pumped full of cytotoxic drugs, that go everywhere in the body, with the hope that enough of the drugs reach the cancer cells and target their nuclear DNA to damage it or destroy the cell. Not only do chemotherapeutic techniques have a range of often serious side effects, mainly affecting all the fast-dividing cells of the body, it also has been shown that often less than 1% of the administered drug molecules enter tumor cells and bind to the nuclear DNA. Another complication is drug resistance of cancer cells. This actually is one of the main causes of failure in the treatment of cancer. Dividing cancer cells acquire genetic changes at a high rate, which means that the cells in a tumor that are resistant to a particular drug will survive and multiply. The result is the re-growth of a tumor that is not sensitive to the original drug. Cancer researchers are looking to nanoparticles as a drug carrier capable of localizing and directly releasing drugs into the cell nucleus, thereby circumventing the multidrug-resistance and intracellular drug-resistance mechanisms to effectively deliver drugs to the vicinity of DNA, leading to a high therapeutic efficacy. Scientists now have developed nanoparticles capable of localizing into the nucleus, giving hope to a much more effective cancer chemotherapy that allows to pinpoint individual cells.
In chemotherapy doctors are using a carpet bombing approach to destroy cancer cells: the patient is pumped full of cytotoxic drugs, that go everywhere in the body, with the hope that enough of the drugs reach the cancer cells and target their nuclear DNA to damage it or destroy the cell. Not only do chemotherapeutic techniques have a range of often serious side effects, mainly affecting all the fast-dividing cells of the body, it also has been shown that often less than 1% of the administered drug molecules enter tumor cells and bind to the nuclear DNA. Another complication is drug resistance of cancer cells. This actually is one of the main causes of failure in the treatment of cancer. Dividing cancer cells acquire genetic changes at a high rate, which means that the cells in a tumor that are resistant to a particular drug will survive and multiply. The result is the re-growth of a tumor that is not sensitive to the original drug. Cancer researchers are looking to nanoparticles as a drug carrier capable of localizing and directly releasing drugs into the cell nucleus, thereby circumventing the multidrug-resistance and intracellular drug-resistance mechanisms to effectively deliver drugs to the vicinity of DNA, leading to a high therapeutic efficacy. Scientists now have developed nanoparticles capable of localizing into the nucleus, giving hope to a much more effective cancer chemotherapy that allows to pinpoint individual cells.
Jun 22nd, 2007
 A lot of buzz has been created by the term "green nanotechnology". In a broad sense, this term includes a wide range of possible applications, from nanotechnology-enabled, environmentally friendly manufacturing processes that reduce waste products (ultimately leading to atomically precise molecular manufacturing with zero waste); the use of nanomaterials as catalysts for greater efficiency in current manufacturing processes by minimizing or eliminating the use of toxic materials (green chemistry principles); the use of nanomaterials and nanodevices to reduce pollution (e.g. water and air filters); and the use of nanomaterials for more efficient alternative energy production (e.g. solar and fuel cells). Unfortunately, there is a flip side to these benefits. As scientists experiment with the development of new chemical or physical methods to produce nanomaterials, the concern for a negative impact on the environment is also heightened: some of the chemical procedures involved in the synthesis of nanomaterials use toxic solvents, could potentially generate hazardous byproducts, and often involve high energy consumption (not to mention the unsolved issue of the potential toxicity of certain nanomaterials). This is leading to a growing awareness of the need to develop clean, nontoxic and environmentally friendly procedures for synthesis and assembly of nanoparticles. Scientists are now exploring the use of biological organisms to literally grow nanomaterials.
A lot of buzz has been created by the term "green nanotechnology". In a broad sense, this term includes a wide range of possible applications, from nanotechnology-enabled, environmentally friendly manufacturing processes that reduce waste products (ultimately leading to atomically precise molecular manufacturing with zero waste); the use of nanomaterials as catalysts for greater efficiency in current manufacturing processes by minimizing or eliminating the use of toxic materials (green chemistry principles); the use of nanomaterials and nanodevices to reduce pollution (e.g. water and air filters); and the use of nanomaterials for more efficient alternative energy production (e.g. solar and fuel cells). Unfortunately, there is a flip side to these benefits. As scientists experiment with the development of new chemical or physical methods to produce nanomaterials, the concern for a negative impact on the environment is also heightened: some of the chemical procedures involved in the synthesis of nanomaterials use toxic solvents, could potentially generate hazardous byproducts, and often involve high energy consumption (not to mention the unsolved issue of the potential toxicity of certain nanomaterials). This is leading to a growing awareness of the need to develop clean, nontoxic and environmentally friendly procedures for synthesis and assembly of nanoparticles. Scientists are now exploring the use of biological organisms to literally grow nanomaterials.
Jun 21st, 2007
 The U.S. Department of Defense (DoD) has released its 2007 review of DoD nanotechnology programs. In 2007, estimated DoD nanotechnology expenditures will be $417m, about the same level as the year before. For the first time, however, the report lists the congressional additions to DoD's investment in nanotechnology. From 2005 to 2007, the Pentagon has requested about $350 million each year for its nanotechnology research. Congressional earmarks of $75.6 million in 2006 (actual) and $63 million in 2007 (estimate) have substantially increased this budget and given the Pentagon more money - and programs - than it actually asked for. The Pentagon report even states that "Congressional additions significantly complicate the assessment of current and proposed funding levels for the DoD investment in nanotechnology, since these Congressional appropriated programs commonly avoid the standard agency technical scrutiny. Furthermore, Congressional additions are often inconsistent with, or even in direct opposition to, the technical focus areas and directions of DoD agencies." Makes you almost feel sorry for the military... but it is yet another perfect example of how warped (sorry, can't use a stronger word here) the U.S. Congressional budget system has become.
The U.S. Department of Defense (DoD) has released its 2007 review of DoD nanotechnology programs. In 2007, estimated DoD nanotechnology expenditures will be $417m, about the same level as the year before. For the first time, however, the report lists the congressional additions to DoD's investment in nanotechnology. From 2005 to 2007, the Pentagon has requested about $350 million each year for its nanotechnology research. Congressional earmarks of $75.6 million in 2006 (actual) and $63 million in 2007 (estimate) have substantially increased this budget and given the Pentagon more money - and programs - than it actually asked for. The Pentagon report even states that "Congressional additions significantly complicate the assessment of current and proposed funding levels for the DoD investment in nanotechnology, since these Congressional appropriated programs commonly avoid the standard agency technical scrutiny. Furthermore, Congressional additions are often inconsistent with, or even in direct opposition to, the technical focus areas and directions of DoD agencies." Makes you almost feel sorry for the military... but it is yet another perfect example of how warped (sorry, can't use a stronger word here) the U.S. Congressional budget system has become.
Jun 20th, 2007
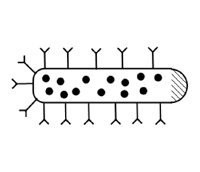 The ideal drug carrier may be something out of science fiction. In principle, it is injected into the body and transports itself to the correct target, such as a tumor, and delivers the required dose at this target. This idealized concept was first proposed by Paul Ehrlich at the beginning of the 20th century and was nicknamed the "magic bullet" concept. With the advent of nanotechnology and nanomedicine this dream is rapidly becoming a reality. Nanotechnology has already been applied to drug delivery and cosmetics through the use of liposomal technology, and now nanoparticles and nanotubes present an exciting and more promising alternative.
The ideal drug carrier may be something out of science fiction. In principle, it is injected into the body and transports itself to the correct target, such as a tumor, and delivers the required dose at this target. This idealized concept was first proposed by Paul Ehrlich at the beginning of the 20th century and was nicknamed the "magic bullet" concept. With the advent of nanotechnology and nanomedicine this dream is rapidly becoming a reality. Nanotechnology has already been applied to drug delivery and cosmetics through the use of liposomal technology, and now nanoparticles and nanotubes present an exciting and more promising alternative.
Jun 19th, 2007
 Biomarkers are of increasing importance in modern medicine for the purpose of early detection and diagnosis of a disease, for instance cancer. Biomarkers are mostly protein molecules that can be measured in blood, other body fluids, and tissues to assess the presence or state of a disease. For example, the presence of an antibody may indicate an infection or an antigen, such as PSA, might indicate the presence of prostate-specific cancer cells. Although protein-based approaches to early detection and diagnosis of cancer have a clear advantage over other, more invasive, methods, protein detection is a challenging problem owing to the structural diversity and complexity of the target analytes. State-of-the-art detection methods have limited application due to their high production cost and instability. Another limitation of current proteomic diagnostics is the limitation of arrays to one or a few markers only; in other words, you can only test for the specific markers that you are looking for and not generally measure levels of proteins in your blood in order to detect anomalies. A novel nanotechnology based protein detector array could change that.
Biomarkers are of increasing importance in modern medicine for the purpose of early detection and diagnosis of a disease, for instance cancer. Biomarkers are mostly protein molecules that can be measured in blood, other body fluids, and tissues to assess the presence or state of a disease. For example, the presence of an antibody may indicate an infection or an antigen, such as PSA, might indicate the presence of prostate-specific cancer cells. Although protein-based approaches to early detection and diagnosis of cancer have a clear advantage over other, more invasive, methods, protein detection is a challenging problem owing to the structural diversity and complexity of the target analytes. State-of-the-art detection methods have limited application due to their high production cost and instability. Another limitation of current proteomic diagnostics is the limitation of arrays to one or a few markers only; in other words, you can only test for the specific markers that you are looking for and not generally measure levels of proteins in your blood in order to detect anomalies. A novel nanotechnology based protein detector array could change that.
 Subscribe to our Nanotechnology Spotlight feed
Subscribe to our Nanotechnology Spotlight feed





