Showing Spotlights 2441 - 2448 of 2838 in category All (newest first):
 Back in the early 1800's it was observed that certain chemicals can speed up a chemical reaction - a process that became known as catalysis and that has become the foundation of the modern chemical industry. By some estimates 90% of all commercially produced chemical products involve catalysts at some stage in the process of their manufacture. Catalysis is the acceleration of a chemical reaction by means of a substance, called a catalyst, which is itself not consumed by the overall reaction. The most effective catalysts are usually transition metals or transition metal complexes. An everyday example of catalysis is the catalytic converter in your car which is used to reduce the toxicity of emissions from your car's engine. Here the catalysts are platinum and manganese which for instance convert harmful nitrogen oxides into harmless nitrogen and oxygen. Since catalysts provide a surface for the chemical reaction to take place on, nanoparticles with their extremely large surface area have become much researched as catalysts (as particles get smaller the larger their surface to volume ratio becomes). Especially in heterogeneous catalysis - where the catalyst is in a different phase (ie. solid, liquid and gas) to the reactants, and that is largely influenced by surface properties - use of nanoscale catalysts opens up a number of possibilities of improving catalytic activity and selectivity. Unfortunately, heterogeneous catalysts supported on a carrier prepared using traditional methods (e.g., impregnation) suffer from a number of problems, such as particle aggregation during preparation, sintering during use (especially at high temperatures), and catalyst leaching because of solvent or pressure drop. This is associated with the poor contact of the catalyst particle with the support surface. A new method of catalyst preparation coming out of Singapore may offer a new concept for catalyst optimization.
Back in the early 1800's it was observed that certain chemicals can speed up a chemical reaction - a process that became known as catalysis and that has become the foundation of the modern chemical industry. By some estimates 90% of all commercially produced chemical products involve catalysts at some stage in the process of their manufacture. Catalysis is the acceleration of a chemical reaction by means of a substance, called a catalyst, which is itself not consumed by the overall reaction. The most effective catalysts are usually transition metals or transition metal complexes. An everyday example of catalysis is the catalytic converter in your car which is used to reduce the toxicity of emissions from your car's engine. Here the catalysts are platinum and manganese which for instance convert harmful nitrogen oxides into harmless nitrogen and oxygen. Since catalysts provide a surface for the chemical reaction to take place on, nanoparticles with their extremely large surface area have become much researched as catalysts (as particles get smaller the larger their surface to volume ratio becomes). Especially in heterogeneous catalysis - where the catalyst is in a different phase (ie. solid, liquid and gas) to the reactants, and that is largely influenced by surface properties - use of nanoscale catalysts opens up a number of possibilities of improving catalytic activity and selectivity. Unfortunately, heterogeneous catalysts supported on a carrier prepared using traditional methods (e.g., impregnation) suffer from a number of problems, such as particle aggregation during preparation, sintering during use (especially at high temperatures), and catalyst leaching because of solvent or pressure drop. This is associated with the poor contact of the catalyst particle with the support surface. A new method of catalyst preparation coming out of Singapore may offer a new concept for catalyst optimization.
Sep 24th, 2007
 Just kidding - I always wanted to write a tabloid headline like that! In case you are expecting a story on the mysteries of crop circles caused by alien nanotechnology - stop reading right here; but the analogy is just too striking when you look at the amazing images coming out of the labs at the University of Southern California, where they developed a new technique to create three-dimensional carbon nanotube structures. While carbon nanotubes possess many exceptional properties which far exceed most known bulk materials, creating controlled nanotube (CNT) microstructures has always been a challenge. Overcoming this challenge is going to be key in developing useful and commercially viable CNT devices. Existing techniques for patterning three-dimensional CNT structures are based on the bottom-up growth of multiwalled CNTs (MWCNTs) from a patterned catalyst, which is limited to 2D-like geometries. Other, complex 3D microstructures have been fabricated with polymer-based resin materials but not with CNTs. The new technique developed by researchers in California uses a focused laser beam to selectively burn local regions of a dense forest of MWCNTs. This technique enables chemically sensitive fields to take advantage of nanotubes' exceptional properties and expand their possible applications into new areas.
Just kidding - I always wanted to write a tabloid headline like that! In case you are expecting a story on the mysteries of crop circles caused by alien nanotechnology - stop reading right here; but the analogy is just too striking when you look at the amazing images coming out of the labs at the University of Southern California, where they developed a new technique to create three-dimensional carbon nanotube structures. While carbon nanotubes possess many exceptional properties which far exceed most known bulk materials, creating controlled nanotube (CNT) microstructures has always been a challenge. Overcoming this challenge is going to be key in developing useful and commercially viable CNT devices. Existing techniques for patterning three-dimensional CNT structures are based on the bottom-up growth of multiwalled CNTs (MWCNTs) from a patterned catalyst, which is limited to 2D-like geometries. Other, complex 3D microstructures have been fabricated with polymer-based resin materials but not with CNTs. The new technique developed by researchers in California uses a focused laser beam to selectively burn local regions of a dense forest of MWCNTs. This technique enables chemically sensitive fields to take advantage of nanotubes' exceptional properties and expand their possible applications into new areas.
Sep 21st, 2007
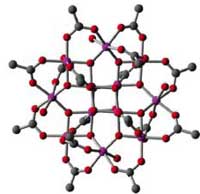 Lithium-ion (Li-ion) batteries are a type of rechargeable battery commonly used in consumer electronics. They are currently one of the most popular types of battery for portable electronics, with one of the best capacity-to-weight ratios, no memory effect, and a slow loss of charge when not in use. Lithium is useful in batteries because of its lightness (it is the lightest metal) and because of the high voltage of the redox reaction between Li and Li+. In lithium ion batteries, a layered compound - lithium copper oxide or or lithium nickel oxide - is utilized as a cathode. Although this material can provide high capacity, its charging/discharging rates are slow because these processes include the absorption/desorption of lithium in the cathode. Recently, organic radical batteries have been developed as a new type of rechargeable battery, in which organic radical polymers are utilized as a cathode active material. They achieved a very fast chargeable/dischargeable rate, though their capacities are lower than those of the lithium ion batteries. A lot of research has gone into fabricating lithium batteries that achieve both high capacity and fast charging/discharging. Researchers in Japan came up with a completely new idea - the molecular cluster battery - where the cathode active material is a well-known manganese molecular cluster that is stable and insoluble to most solvents and exhibits a multi-step redox reaction. Although the battery was rechargeable, in early experiments the fast charging�??discharging was not yet achieved due to the chemical decomposition of the cluster. Nevertheless, this is a first step that opens up a new branch of research into high-performance rechargeable molecular cluster batteries.
Lithium-ion (Li-ion) batteries are a type of rechargeable battery commonly used in consumer electronics. They are currently one of the most popular types of battery for portable electronics, with one of the best capacity-to-weight ratios, no memory effect, and a slow loss of charge when not in use. Lithium is useful in batteries because of its lightness (it is the lightest metal) and because of the high voltage of the redox reaction between Li and Li+. In lithium ion batteries, a layered compound - lithium copper oxide or or lithium nickel oxide - is utilized as a cathode. Although this material can provide high capacity, its charging/discharging rates are slow because these processes include the absorption/desorption of lithium in the cathode. Recently, organic radical batteries have been developed as a new type of rechargeable battery, in which organic radical polymers are utilized as a cathode active material. They achieved a very fast chargeable/dischargeable rate, though their capacities are lower than those of the lithium ion batteries. A lot of research has gone into fabricating lithium batteries that achieve both high capacity and fast charging/discharging. Researchers in Japan came up with a completely new idea - the molecular cluster battery - where the cathode active material is a well-known manganese molecular cluster that is stable and insoluble to most solvents and exhibits a multi-step redox reaction. Although the battery was rechargeable, in early experiments the fast charging�??discharging was not yet achieved due to the chemical decomposition of the cluster. Nevertheless, this is a first step that opens up a new branch of research into high-performance rechargeable molecular cluster batteries.
Sep 20th, 2007
 Probably any chemist must have dreamt about it: Quick isolation of a chemical from a reaction mixture without the hassle of tedious liquid handling lasting for hours. The problem is that today the product separation and postprocessing of organic compounds, proteins, nucleic acids, and natural products from complex reaction mixtures remains labor-intensive and costly. Catalytic processes in the liquid phase are important in many areas of the fine and specialty chemicals industries, and the use of solid catalysts means easier catalyst separation and recovery, hence facilitating their reuse. Usually a smaller catalyst particles means a higher activity, and sub micron particles are particularly attractive because they experience no significant attrition, i.e. no reduction in particle size. A major difficultly with small particles is the cumbersome fact that they are almost impossible to separate by conventional means, which can lead to the blocking of filters and valves by the catalyst. A possible solution to this problem is the magnetic separation of products from mixtures, as routinely applied in biochemistry. Unfortunately, the exorbitant price of magnetic microbeads and their low binding capacity limit their use for organic synthesis. Researchers in Switzerland, have now found a way to link organic molecules to metallic nanomagnets. This allows separating tagged molecules or reagents after synthesis within seconds. The technology is now explored in organic chemistry and biotechnology as an alternative to chromatography or crystallization. Combining classical organic synthesis or polymer production with magnetic separation could potentially revolutionize key processes in the chemical industry.
Probably any chemist must have dreamt about it: Quick isolation of a chemical from a reaction mixture without the hassle of tedious liquid handling lasting for hours. The problem is that today the product separation and postprocessing of organic compounds, proteins, nucleic acids, and natural products from complex reaction mixtures remains labor-intensive and costly. Catalytic processes in the liquid phase are important in many areas of the fine and specialty chemicals industries, and the use of solid catalysts means easier catalyst separation and recovery, hence facilitating their reuse. Usually a smaller catalyst particles means a higher activity, and sub micron particles are particularly attractive because they experience no significant attrition, i.e. no reduction in particle size. A major difficultly with small particles is the cumbersome fact that they are almost impossible to separate by conventional means, which can lead to the blocking of filters and valves by the catalyst. A possible solution to this problem is the magnetic separation of products from mixtures, as routinely applied in biochemistry. Unfortunately, the exorbitant price of magnetic microbeads and their low binding capacity limit their use for organic synthesis. Researchers in Switzerland, have now found a way to link organic molecules to metallic nanomagnets. This allows separating tagged molecules or reagents after synthesis within seconds. The technology is now explored in organic chemistry and biotechnology as an alternative to chromatography or crystallization. Combining classical organic synthesis or polymer production with magnetic separation could potentially revolutionize key processes in the chemical industry.
Sep 19th, 2007
 There is a huge demand for medical implants for almost every body part you can think of. As we have reported here before, the market for medical implant devices in the U.S. alone is estimated to be $23 billion per year and it is expected to grow by about 10% annually for the next few years. Implantable cardioverter defibrillators, cardiac resynchronization therapy devices, pacemakers, tissue and spinal orthopedic implants, hip replacements, phakic intraocular lenses and cosmetic implants will be among the top sellers. Current medical implants, such as orthopedic implants and heart valves, are made of titanium and stainless steel alloys, primarily because they are biocompatible. Unfortunately, in many cases these metal alloys with a life span of 10-15 years may wear out within the lifetime of the patient. With recent advances in industrial synthesis of diamond and diamond-like carbon film bringing prices down significantly, researchers are increasingly experimenting with diamond coatings for medical implants. On the upside, the wear resistance of diamond is dramatically superior to titanium and stainless steel. On the downside, because it attracts coagulating proteins, its blood clotting response is slightly worse than these materials and the possibility has been raised that nanostructured surface features of diamond might abrade tissue. That's not something you necessarily want to have in your artificial knee or hip joints (although some of the currently used implant materials cause problems as well). Researchers have now run simulations that show that thin layers of ice could persist on specially treated diamond coatings at temperatures well above body temperature. The soft and hydrophilic ice multilayers might enable diamond-coated medical devices that reduce abrasion and are highly resistant to protein absorption.
There is a huge demand for medical implants for almost every body part you can think of. As we have reported here before, the market for medical implant devices in the U.S. alone is estimated to be $23 billion per year and it is expected to grow by about 10% annually for the next few years. Implantable cardioverter defibrillators, cardiac resynchronization therapy devices, pacemakers, tissue and spinal orthopedic implants, hip replacements, phakic intraocular lenses and cosmetic implants will be among the top sellers. Current medical implants, such as orthopedic implants and heart valves, are made of titanium and stainless steel alloys, primarily because they are biocompatible. Unfortunately, in many cases these metal alloys with a life span of 10-15 years may wear out within the lifetime of the patient. With recent advances in industrial synthesis of diamond and diamond-like carbon film bringing prices down significantly, researchers are increasingly experimenting with diamond coatings for medical implants. On the upside, the wear resistance of diamond is dramatically superior to titanium and stainless steel. On the downside, because it attracts coagulating proteins, its blood clotting response is slightly worse than these materials and the possibility has been raised that nanostructured surface features of diamond might abrade tissue. That's not something you necessarily want to have in your artificial knee or hip joints (although some of the currently used implant materials cause problems as well). Researchers have now run simulations that show that thin layers of ice could persist on specially treated diamond coatings at temperatures well above body temperature. The soft and hydrophilic ice multilayers might enable diamond-coated medical devices that reduce abrasion and are highly resistant to protein absorption.
Sep 18th, 2007
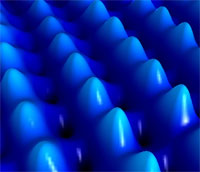 Apart from buying a new computer every year it seems you need to upgrade your old machine on a regular basis to keep pace with ever bigger software packages and image files. Apart from the hassle of having to perform major surgery on your computer, these upgrades cost money. But, what if these upgrades were no longer necessary? What if your desktop computer came standard with the ability to store more data than you could ever possibly need and was able to function at unbelievable speeds? This would be too good to be true, right? Besides, who has the space for such a megacomputer. Well, imagine that this megacomputer could be packaged as a smaller device than current laptops, and cost only a fraction of today�??s prices? This sounds like hard core science fiction, but like so many radical science fiction ideas - the real thing might happen sooner than you think. As chip designers are nearing the physical limits of Moore's law (some say that the exponential increase in the cost of semiconductor production will most likely stop the current miniaturization trend before its physical limits are reached), scientists around the globe are working hard on developing the field of molecular electronics. An interdisciplinary science that includes physics, chemistry, nanotechnology, materials science and even biology, molecular electronics involves using molecular building blocks in the manufacture of electronic components. Driven by a growing interest in alternative concepts, like the integration of molecules as carriers of an electronic function, the electronics industry is poised to take the crucial step of integrating molecular devices into electronic circuits.
Apart from buying a new computer every year it seems you need to upgrade your old machine on a regular basis to keep pace with ever bigger software packages and image files. Apart from the hassle of having to perform major surgery on your computer, these upgrades cost money. But, what if these upgrades were no longer necessary? What if your desktop computer came standard with the ability to store more data than you could ever possibly need and was able to function at unbelievable speeds? This would be too good to be true, right? Besides, who has the space for such a megacomputer. Well, imagine that this megacomputer could be packaged as a smaller device than current laptops, and cost only a fraction of today�??s prices? This sounds like hard core science fiction, but like so many radical science fiction ideas - the real thing might happen sooner than you think. As chip designers are nearing the physical limits of Moore's law (some say that the exponential increase in the cost of semiconductor production will most likely stop the current miniaturization trend before its physical limits are reached), scientists around the globe are working hard on developing the field of molecular electronics. An interdisciplinary science that includes physics, chemistry, nanotechnology, materials science and even biology, molecular electronics involves using molecular building blocks in the manufacture of electronic components. Driven by a growing interest in alternative concepts, like the integration of molecules as carriers of an electronic function, the electronics industry is poised to take the crucial step of integrating molecular devices into electronic circuits.
Sep 17th, 2007
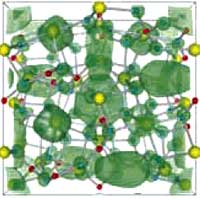 Many of us don't like to admit it, but televisions are an important part of our lives. Technology has improved the quality and convenience of TVs and has given us a whole new set of choices - high definition, plasma, and liquid crystal displays. With an estimated 66 million sets to be sold this year, flat-screen LCD televisions are the most popular choice. Much of the reason behind the popularity of these high-tech wonders is the decrease in price that happens with most cool technology - a few years after they have been on the market. But, for the popular LCD TV, a shortage of a key compound used to produce LCDs may force manufacturers to raise prices. Electronics suppliers are facing a shortage of the rare metal indium, a co-product of zinc mining. Indium is a rare, malleable and easily fused metal, similar to aluminum, which is used to make indium tin oxide (ITO), the standard transparent electrode used in nearly all flat panel displays and microdisplays. Indium is expensive and scarce and demand is increasing. According to Displaybank, the demand for indium was 861 tons in 2006 and may reach nearly 2000 tons by 2011. Five years ago, indium was about $100/ kg; now it costs $800/kg. Displaybank expects the total sales of indium in 2007 to be $533 million. But, geologists say the cost of indium may not matter soon, because the earth's supply of it could be gone in four years. This could put a serious damper on that 52" LCD screen you've been dreaming about. Fortunately, with the help of nanotechnology, a team of scientists in Japan have developed a new material that may replace the need for indium in LCD production.
Many of us don't like to admit it, but televisions are an important part of our lives. Technology has improved the quality and convenience of TVs and has given us a whole new set of choices - high definition, plasma, and liquid crystal displays. With an estimated 66 million sets to be sold this year, flat-screen LCD televisions are the most popular choice. Much of the reason behind the popularity of these high-tech wonders is the decrease in price that happens with most cool technology - a few years after they have been on the market. But, for the popular LCD TV, a shortage of a key compound used to produce LCDs may force manufacturers to raise prices. Electronics suppliers are facing a shortage of the rare metal indium, a co-product of zinc mining. Indium is a rare, malleable and easily fused metal, similar to aluminum, which is used to make indium tin oxide (ITO), the standard transparent electrode used in nearly all flat panel displays and microdisplays. Indium is expensive and scarce and demand is increasing. According to Displaybank, the demand for indium was 861 tons in 2006 and may reach nearly 2000 tons by 2011. Five years ago, indium was about $100/ kg; now it costs $800/kg. Displaybank expects the total sales of indium in 2007 to be $533 million. But, geologists say the cost of indium may not matter soon, because the earth's supply of it could be gone in four years. This could put a serious damper on that 52" LCD screen you've been dreaming about. Fortunately, with the help of nanotechnology, a team of scientists in Japan have developed a new material that may replace the need for indium in LCD production.
Sep 14th, 2007
 New technology, whether it is a novel cancer treatment or an innovative approach to making a new material, almost always comes with risk. Nanotechnologies are no different. Certain nano-fabrication techniques employ toxic chemicals, the production of carbon nanotubes results in dangerous byproducts, and the big question as to what degree certain engineered nanoparticles could be harmful to humans and the environment has not been answered yet. The potentially adverse health effects of fine and ultrafine particles have been studied for decades. However, at the core of the nanotoxicological debate is the fact that nanoparticles are not just a smaller version of certain particles, but they are very different from their everyday counterparts with regard to their physical properties and catalytic activities. Thus their adverse effects cannot simply be derived from the known toxicity of the macro-sized material. One useful contribution to moving the nanotoxicology discussion further along came from the 1st Nobel Forum Mini-Symposium on Nanotoxicology that was held in Stockholm, Sweden. The event's program was devoted to the topic of definitions and standardization in nanotoxicological research, as well as nano-specific risk assessment and regulatory/legislative issues. A group of international experts presented examples of recent and ongoing studies of carbon-based nanomaterials, including single-walled carbon nanotubes, using a wide range of in vitro and in vivo model systems. This Spotlight will provide you with some highlights and conclusions from this exciting meeting.
New technology, whether it is a novel cancer treatment or an innovative approach to making a new material, almost always comes with risk. Nanotechnologies are no different. Certain nano-fabrication techniques employ toxic chemicals, the production of carbon nanotubes results in dangerous byproducts, and the big question as to what degree certain engineered nanoparticles could be harmful to humans and the environment has not been answered yet. The potentially adverse health effects of fine and ultrafine particles have been studied for decades. However, at the core of the nanotoxicological debate is the fact that nanoparticles are not just a smaller version of certain particles, but they are very different from their everyday counterparts with regard to their physical properties and catalytic activities. Thus their adverse effects cannot simply be derived from the known toxicity of the macro-sized material. One useful contribution to moving the nanotoxicology discussion further along came from the 1st Nobel Forum Mini-Symposium on Nanotoxicology that was held in Stockholm, Sweden. The event's program was devoted to the topic of definitions and standardization in nanotoxicological research, as well as nano-specific risk assessment and regulatory/legislative issues. A group of international experts presented examples of recent and ongoing studies of carbon-based nanomaterials, including single-walled carbon nanotubes, using a wide range of in vitro and in vivo model systems. This Spotlight will provide you with some highlights and conclusions from this exciting meeting.
Sep 13th, 2007
 Back in the early 1800's it was observed that certain chemicals can speed up a chemical reaction - a process that became known as catalysis and that has become the foundation of the modern chemical industry. By some estimates 90% of all commercially produced chemical products involve catalysts at some stage in the process of their manufacture. Catalysis is the acceleration of a chemical reaction by means of a substance, called a catalyst, which is itself not consumed by the overall reaction. The most effective catalysts are usually transition metals or transition metal complexes. An everyday example of catalysis is the catalytic converter in your car which is used to reduce the toxicity of emissions from your car's engine. Here the catalysts are platinum and manganese which for instance convert harmful nitrogen oxides into harmless nitrogen and oxygen. Since catalysts provide a surface for the chemical reaction to take place on, nanoparticles with their extremely large surface area have become much researched as catalysts (as particles get smaller the larger their surface to volume ratio becomes). Especially in heterogeneous catalysis - where the catalyst is in a different phase (ie. solid, liquid and gas) to the reactants, and that is largely influenced by surface properties - use of nanoscale catalysts opens up a number of possibilities of improving catalytic activity and selectivity. Unfortunately, heterogeneous catalysts supported on a carrier prepared using traditional methods (e.g., impregnation) suffer from a number of problems, such as particle aggregation during preparation, sintering during use (especially at high temperatures), and catalyst leaching because of solvent or pressure drop. This is associated with the poor contact of the catalyst particle with the support surface. A new method of catalyst preparation coming out of Singapore may offer a new concept for catalyst optimization.
Back in the early 1800's it was observed that certain chemicals can speed up a chemical reaction - a process that became known as catalysis and that has become the foundation of the modern chemical industry. By some estimates 90% of all commercially produced chemical products involve catalysts at some stage in the process of their manufacture. Catalysis is the acceleration of a chemical reaction by means of a substance, called a catalyst, which is itself not consumed by the overall reaction. The most effective catalysts are usually transition metals or transition metal complexes. An everyday example of catalysis is the catalytic converter in your car which is used to reduce the toxicity of emissions from your car's engine. Here the catalysts are platinum and manganese which for instance convert harmful nitrogen oxides into harmless nitrogen and oxygen. Since catalysts provide a surface for the chemical reaction to take place on, nanoparticles with their extremely large surface area have become much researched as catalysts (as particles get smaller the larger their surface to volume ratio becomes). Especially in heterogeneous catalysis - where the catalyst is in a different phase (ie. solid, liquid and gas) to the reactants, and that is largely influenced by surface properties - use of nanoscale catalysts opens up a number of possibilities of improving catalytic activity and selectivity. Unfortunately, heterogeneous catalysts supported on a carrier prepared using traditional methods (e.g., impregnation) suffer from a number of problems, such as particle aggregation during preparation, sintering during use (especially at high temperatures), and catalyst leaching because of solvent or pressure drop. This is associated with the poor contact of the catalyst particle with the support surface. A new method of catalyst preparation coming out of Singapore may offer a new concept for catalyst optimization.
 Subscribe to our Nanotechnology Spotlight feed
Subscribe to our Nanotechnology Spotlight feed





