Showing Spotlights 2449 - 2456 of 2838 in category All (newest first):
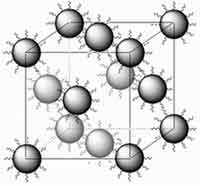 Zinc Oxide (ZnO) has long been used in its powdered form as pigments in paints, coatings for papers, in the commercial manufacture of rubber goods as well as UVA and UVB blocker and mild antimicrobial in cosmetics. ZnO is also one of the most important semiconductor compounds and numerous reports have been documented in the literature about the preparation and characterization of ZnO nanocrystals. While polycrystalline forms of ZnO have been used for technical uses such as piezoelectric transducers, light emitting diodes, and transparent conducting films, the progress in developing single crystal bulk ZnO have brought its promise as a wide band gap semiconductor to the fore. Superstructures formed from ZnO nanocrystal quantum dots may find applications in various areas such as optics, electronics and magnetism. For these 2D and 3D superstructures to be useful they need to be well-ordered. Usually, nanocrystals without any surface modification are less stable and they usually undergo aggregation or crystal growth, and consequently it is rather hard for bare nanocrystals to self-assemble into 2D, and especially into 3D, ordered structures. So far, most well-ordered assemblies of nanocrystals have been prepared through a surface modification approach. Efforts have been made to prepare superstructures composed of ZnO nanocrystals but it is rather challenging to obtain well-ordered 3D ZnO superlattices. Researchers in China have now found that ZnO nanocrystals capped with ionic liquids spontaneously assemble into a three-dimensional lattice. Apparently, simply drying a solution of the modified ZnO nanocrystals is all that is needed for the superlattice to form. The presence of the ionic liquid prevents the nanocrystals from aggregating.
Zinc Oxide (ZnO) has long been used in its powdered form as pigments in paints, coatings for papers, in the commercial manufacture of rubber goods as well as UVA and UVB blocker and mild antimicrobial in cosmetics. ZnO is also one of the most important semiconductor compounds and numerous reports have been documented in the literature about the preparation and characterization of ZnO nanocrystals. While polycrystalline forms of ZnO have been used for technical uses such as piezoelectric transducers, light emitting diodes, and transparent conducting films, the progress in developing single crystal bulk ZnO have brought its promise as a wide band gap semiconductor to the fore. Superstructures formed from ZnO nanocrystal quantum dots may find applications in various areas such as optics, electronics and magnetism. For these 2D and 3D superstructures to be useful they need to be well-ordered. Usually, nanocrystals without any surface modification are less stable and they usually undergo aggregation or crystal growth, and consequently it is rather hard for bare nanocrystals to self-assemble into 2D, and especially into 3D, ordered structures. So far, most well-ordered assemblies of nanocrystals have been prepared through a surface modification approach. Efforts have been made to prepare superstructures composed of ZnO nanocrystals but it is rather challenging to obtain well-ordered 3D ZnO superlattices. Researchers in China have now found that ZnO nanocrystals capped with ionic liquids spontaneously assemble into a three-dimensional lattice. Apparently, simply drying a solution of the modified ZnO nanocrystals is all that is needed for the superlattice to form. The presence of the ionic liquid prevents the nanocrystals from aggregating.
Sep 12th, 2007
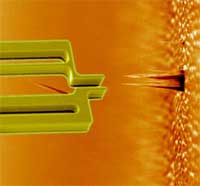 Hands-on nanotechnology: towards a nanorobotic assembly line (Nanowerk Spotlight) Until the twentieth century, a single craftsman or team of craftsmen would normally create each part of an industrial product individually and assemble them together into a single item, making changes in the parts so that they would fit together and work together; the so-called English System of manufacture. Then Henry Ford came along and in 1907-08 developed the assembly line for his Model T automobile. This innovation revolutionized not only industry but also our society because it allowed mass production of industrial goods at much lower cost than before. At its core, an assembly line is a manufacturing process in which interchangeable parts are added to a product in a sequential manner to create a finished product. Nanotechnology techniques today are pretty much where the industrial world was before Ford's assembly line - a domain of craftsmen and not of mass production. It has long been a dream for nanotechnologists that robots could one day be used in a similar way to produce nanodevices. A group of researchers from Denmark and Germany have now developed the rudimentary beginnings of the nanotechnology equivalent of an assembly line. They have shown 'pick-and-place' assembly of a working device using a silicon gripper - a robotic 'hand' some 10000 times smaller than a human hand. This nanogripper, controlled by a nanorobotic arm, is capable of picking up a carbon nanofiber (CN) and fix it onto the tip of an atomic force microscope cantilever.
Hands-on nanotechnology: towards a nanorobotic assembly line (Nanowerk Spotlight) Until the twentieth century, a single craftsman or team of craftsmen would normally create each part of an industrial product individually and assemble them together into a single item, making changes in the parts so that they would fit together and work together; the so-called English System of manufacture. Then Henry Ford came along and in 1907-08 developed the assembly line for his Model T automobile. This innovation revolutionized not only industry but also our society because it allowed mass production of industrial goods at much lower cost than before. At its core, an assembly line is a manufacturing process in which interchangeable parts are added to a product in a sequential manner to create a finished product. Nanotechnology techniques today are pretty much where the industrial world was before Ford's assembly line - a domain of craftsmen and not of mass production. It has long been a dream for nanotechnologists that robots could one day be used in a similar way to produce nanodevices. A group of researchers from Denmark and Germany have now developed the rudimentary beginnings of the nanotechnology equivalent of an assembly line. They have shown 'pick-and-place' assembly of a working device using a silicon gripper - a robotic 'hand' some 10000 times smaller than a human hand. This nanogripper, controlled by a nanorobotic arm, is capable of picking up a carbon nanofiber (CN) and fix it onto the tip of an atomic force microscope cantilever.
Sep 11th, 2007
 For the builders and engineers among you, our subject today is cement. Not necessarily a material one would associate with high-tech, not to mention nanotechnology. However, it's probably fair to say that our modern society is built on cement. Look around you and you'll find it everywhere - in buildings, roads, bridges, dams. Early construction cement (the word goes back to the Romans who used the term opus caementitium to describe masonry which resembled concrete and was made from crushed rock with burnt lime as binder) probably is as old as construction itself. So what is it? Cement, as it is commonly known, is a mixture of compounds made by burning limestone and clay together at very high temperatures. Cement is then used, together with water, as binder in a synthetic composite material known as concrete. For concrete to obtain its optimal properties it needs to harden. And that takes time. For builders, time is money and particularly in industrial settings time is a major cost issue. Time is also a safety and convenience factor, think about infrastructure repair work on roads and dams for instance. Cement manufacturers have already known that reducing the particle size of cements results in faster-binding formulations. By taking the ultimate reduction down to the nanoscale, researchers in Switzerland have shown that a one-step preparation of nanoparticulate cement with a conventional Portland cement composition results in a drastically increased early reactivity of the cement.
For the builders and engineers among you, our subject today is cement. Not necessarily a material one would associate with high-tech, not to mention nanotechnology. However, it's probably fair to say that our modern society is built on cement. Look around you and you'll find it everywhere - in buildings, roads, bridges, dams. Early construction cement (the word goes back to the Romans who used the term opus caementitium to describe masonry which resembled concrete and was made from crushed rock with burnt lime as binder) probably is as old as construction itself. So what is it? Cement, as it is commonly known, is a mixture of compounds made by burning limestone and clay together at very high temperatures. Cement is then used, together with water, as binder in a synthetic composite material known as concrete. For concrete to obtain its optimal properties it needs to harden. And that takes time. For builders, time is money and particularly in industrial settings time is a major cost issue. Time is also a safety and convenience factor, think about infrastructure repair work on roads and dams for instance. Cement manufacturers have already known that reducing the particle size of cements results in faster-binding formulations. By taking the ultimate reduction down to the nanoscale, researchers in Switzerland have shown that a one-step preparation of nanoparticulate cement with a conventional Portland cement composition results in a drastically increased early reactivity of the cement.
Sep 10th, 2007
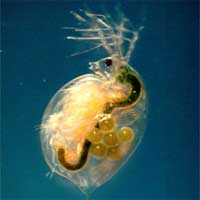 The much heralded nanotechnology revolution is not happening with a big bang that completely turns our lives upside down, but rather in a creeping stealth mode where many ordinary everyday products, from cosmetics and textiles to electronic devices, sporting goods and car paint increasingly contain engineered nanoparticles. Sometimes, these nanoparticles are just a smaller version of the material already used in a product, for instance zinc oxide in sunscreen lotions, sometimes these particles are a new addition to a product, as for example fullerenes added to oil lubricants to improve their performance. This trend of increasing use of engineered nanoparticles in commercial products raises the question of what happens at the end-of-life stage of these products, when they get disposed or recycled. Is there is a risk of these nanoparticles being released into the environment? And if yes, is there a risk of these nanoparticles causing harm? This is an area of nanotechnology risk research that remains largely unexplored. In a groundbreaking study to determine the effects of nanoparticles on aquatic organisms, scientists at the University of Wisconsin's Great Lakes Water Institute in Milwaukee have demonstrated that all nanoparticles are not created equal - at least when it comes to their effects on aquatic organisms. They have also discovered that existing attitudes toward the safety of titanium dioxide may be dangerous.
The much heralded nanotechnology revolution is not happening with a big bang that completely turns our lives upside down, but rather in a creeping stealth mode where many ordinary everyday products, from cosmetics and textiles to electronic devices, sporting goods and car paint increasingly contain engineered nanoparticles. Sometimes, these nanoparticles are just a smaller version of the material already used in a product, for instance zinc oxide in sunscreen lotions, sometimes these particles are a new addition to a product, as for example fullerenes added to oil lubricants to improve their performance. This trend of increasing use of engineered nanoparticles in commercial products raises the question of what happens at the end-of-life stage of these products, when they get disposed or recycled. Is there is a risk of these nanoparticles being released into the environment? And if yes, is there a risk of these nanoparticles causing harm? This is an area of nanotechnology risk research that remains largely unexplored. In a groundbreaking study to determine the effects of nanoparticles on aquatic organisms, scientists at the University of Wisconsin's Great Lakes Water Institute in Milwaukee have demonstrated that all nanoparticles are not created equal - at least when it comes to their effects on aquatic organisms. They have also discovered that existing attitudes toward the safety of titanium dioxide may be dangerous.
Sep 7th, 2007
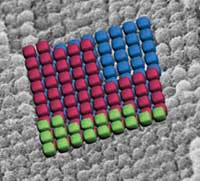 In order to exploit the unique properties of nanoscale materials for advanced applications it is often necessary to assemble nanoparticles into arrays with specific architectures. The interaction among the nanoparticles, or effects arising from their assembled larger structure, could result in interesting optical, magnetic or catalytic properties that researchers and engineers then could exploit for new materials and applications. In recent years, there has been much interest in colloidal crystals - which are examples of periodic nanoparticle arrays - as photonic crystals, templates for photonic crystals, sensors, optical and electrooptical devices, and as model systems to study crystallization processes. The success of many of these potential applications is currently limited by scientists' ability to control the structure of colloidal crystals. Normally, crystallization of uniform colloids produces face-centered cubic or hexagonal close-packing. A few other colloidal crystal structures have recently been reported, but they either require careful balance of electrostatic interactions between colloidal particles, or they rely on directing nanoparticles on a lithographic pattern that then dictates the geometry of a few layers in a thin film. New research now has resulted in a completely different and novel approach of colloidal crystallization that results in simple cubic colloidal crystals extending over many unit cells in three dimensions. Simple cubic packing is quite rare, even in atomic structures. Here, it results from combined disassembly and self-reassembly of a template- directed structure in a single reaction step.
In order to exploit the unique properties of nanoscale materials for advanced applications it is often necessary to assemble nanoparticles into arrays with specific architectures. The interaction among the nanoparticles, or effects arising from their assembled larger structure, could result in interesting optical, magnetic or catalytic properties that researchers and engineers then could exploit for new materials and applications. In recent years, there has been much interest in colloidal crystals - which are examples of periodic nanoparticle arrays - as photonic crystals, templates for photonic crystals, sensors, optical and electrooptical devices, and as model systems to study crystallization processes. The success of many of these potential applications is currently limited by scientists' ability to control the structure of colloidal crystals. Normally, crystallization of uniform colloids produces face-centered cubic or hexagonal close-packing. A few other colloidal crystal structures have recently been reported, but they either require careful balance of electrostatic interactions between colloidal particles, or they rely on directing nanoparticles on a lithographic pattern that then dictates the geometry of a few layers in a thin film. New research now has resulted in a completely different and novel approach of colloidal crystallization that results in simple cubic colloidal crystals extending over many unit cells in three dimensions. Simple cubic packing is quite rare, even in atomic structures. Here, it results from combined disassembly and self-reassembly of a template- directed structure in a single reaction step.
Sep 6th, 2007
 When Gutenberg built his printing machine with moveable type in the mid 15th century, little idea did he have that he started the information age; even less that scientists would adopt the process to the nanoscale. The printing press went through several revolutionary improvements such as Lanston's monotype machine (1884), Mergenthaler's linotype machine (1886), the photo-typesetting process developed in the 1960s and finally digital printing in the 1980s. Today, printing is the most widespread technology to deposit small particles onto various surfaces. Commercial desktop laser printers use toner particles with a few microns in size while top of the line high-priced industrial printing machines sometimes already use sub-micron size particles. On the other hand, the precise positioning of nanoparticles on surfaces is key to most nanotechnology applications especially nanoelectronics. However, for automated patterning of particles, existing methods are either slow (e.g., dip-pen lithography) or require prefabricated patterns on the target substrate (e.g. for electrostatic positioning). Using a process akin to the printing press, researchers already have managed to bypass the need for epitaxial growth or wafer bonding to integrate wide ranging classes of dissimilar semiconducting nanomaterials onto substrates for the purpose of constructing heterogeneous, three dimensional electronics. Scientist in Switzerland have now developed a parallel method for the assembly and integration of a large number of bulk-synthesized nanoparticles onto an unstructured surface with high resolution and yield.
When Gutenberg built his printing machine with moveable type in the mid 15th century, little idea did he have that he started the information age; even less that scientists would adopt the process to the nanoscale. The printing press went through several revolutionary improvements such as Lanston's monotype machine (1884), Mergenthaler's linotype machine (1886), the photo-typesetting process developed in the 1960s and finally digital printing in the 1980s. Today, printing is the most widespread technology to deposit small particles onto various surfaces. Commercial desktop laser printers use toner particles with a few microns in size while top of the line high-priced industrial printing machines sometimes already use sub-micron size particles. On the other hand, the precise positioning of nanoparticles on surfaces is key to most nanotechnology applications especially nanoelectronics. However, for automated patterning of particles, existing methods are either slow (e.g., dip-pen lithography) or require prefabricated patterns on the target substrate (e.g. for electrostatic positioning). Using a process akin to the printing press, researchers already have managed to bypass the need for epitaxial growth or wafer bonding to integrate wide ranging classes of dissimilar semiconducting nanomaterials onto substrates for the purpose of constructing heterogeneous, three dimensional electronics. Scientist in Switzerland have now developed a parallel method for the assembly and integration of a large number of bulk-synthesized nanoparticles onto an unstructured surface with high resolution and yield.
Sep 5th, 2007
 There is a significant and growing need across the research and medical communities for low-cost, high throughput DNA separation and quantification techniques. The isolation of DNA is a prerequisite step for many molecular biology techniques and experiments. Although single molecule techniques afford extremely high sensitivity, to date, such experiments have remained within the confines of academic and research laboratories. The primary reasons for this state of affairs relate to throughput, detection efficiencies and analysis times. For example, in a conventional solution-based single molecule detection experiment, one can only detect approximately 10,000 molecules per minute, or one molecule every 6 milliseconds. While this may sound a lot, consider that a small drop of water (ca. 5 ml) contains approx. 1.67 x 10e23 molecules, that is 1.67 followed by 23 zeros. At that speed you need over 100 trillion years to detect all the water molecules in this single drop. Using a novel nanopore array developed by researchers in the UK, expect to be able to detect up to 1 million molecules simultaneously in the same 6 millisecond time window, representing an improvement in throughput of over six orders of magnitude (and bringing the timeframe for analyzing the molecules in a single water drop down to some 60 billion years - about five to six times the estimated age of the universe).
There is a significant and growing need across the research and medical communities for low-cost, high throughput DNA separation and quantification techniques. The isolation of DNA is a prerequisite step for many molecular biology techniques and experiments. Although single molecule techniques afford extremely high sensitivity, to date, such experiments have remained within the confines of academic and research laboratories. The primary reasons for this state of affairs relate to throughput, detection efficiencies and analysis times. For example, in a conventional solution-based single molecule detection experiment, one can only detect approximately 10,000 molecules per minute, or one molecule every 6 milliseconds. While this may sound a lot, consider that a small drop of water (ca. 5 ml) contains approx. 1.67 x 10e23 molecules, that is 1.67 followed by 23 zeros. At that speed you need over 100 trillion years to detect all the water molecules in this single drop. Using a novel nanopore array developed by researchers in the UK, expect to be able to detect up to 1 million molecules simultaneously in the same 6 millisecond time window, representing an improvement in throughput of over six orders of magnitude (and bringing the timeframe for analyzing the molecules in a single water drop down to some 60 billion years - about five to six times the estimated age of the universe).
Sep 4th, 2007
 You might have come across the acronym NBIC, which stands for Nanotechnology, Biotechnology, Information technology and new technologies based on Cognitive science. Initially introduced in the U.S. National Science Foundation's 'Converging Technologies for Improving Human Performance' report this acronym is often used to describe the basic idea that scientific and technological innovation can be stimulated through the convergence of two, three, or all four fields. At its most radical (and most controversial), proponents of convergence suggest that nanotechnologies will promote the unification of most branches of science and technology, based on the unity of nature at the nanoscale, including cognitive sciences. We'll keep you posted on this over the next few decades and see how it all works out. For the time being, though, it would be nice to be able to report on something more hands-on and - dare I write it - even practical. As it happens, scientists at the University of Toronto have done exactly that. They have demonstrated, for what appears to be the first time, the convergence of nanotechnology, microtechnology, microfluidics, photonics, signal processing, and proteomics to build a medical device that could lead to the development of fast, portable point-of-care diagnostics for infectious disease (IDs) such as HIV, SARS and many others.
You might have come across the acronym NBIC, which stands for Nanotechnology, Biotechnology, Information technology and new technologies based on Cognitive science. Initially introduced in the U.S. National Science Foundation's 'Converging Technologies for Improving Human Performance' report this acronym is often used to describe the basic idea that scientific and technological innovation can be stimulated through the convergence of two, three, or all four fields. At its most radical (and most controversial), proponents of convergence suggest that nanotechnologies will promote the unification of most branches of science and technology, based on the unity of nature at the nanoscale, including cognitive sciences. We'll keep you posted on this over the next few decades and see how it all works out. For the time being, though, it would be nice to be able to report on something more hands-on and - dare I write it - even practical. As it happens, scientists at the University of Toronto have done exactly that. They have demonstrated, for what appears to be the first time, the convergence of nanotechnology, microtechnology, microfluidics, photonics, signal processing, and proteomics to build a medical device that could lead to the development of fast, portable point-of-care diagnostics for infectious disease (IDs) such as HIV, SARS and many others.
Aug 31st, 2007
 Zinc Oxide (ZnO) has long been used in its powdered form as pigments in paints, coatings for papers, in the commercial manufacture of rubber goods as well as UVA and UVB blocker and mild antimicrobial in cosmetics. ZnO is also one of the most important semiconductor compounds and numerous reports have been documented in the literature about the preparation and characterization of ZnO nanocrystals. While polycrystalline forms of ZnO have been used for technical uses such as piezoelectric transducers, light emitting diodes, and transparent conducting films, the progress in developing single crystal bulk ZnO have brought its promise as a wide band gap semiconductor to the fore. Superstructures formed from ZnO nanocrystal quantum dots may find applications in various areas such as optics, electronics and magnetism. For these 2D and 3D superstructures to be useful they need to be well-ordered. Usually, nanocrystals without any surface modification are less stable and they usually undergo aggregation or crystal growth, and consequently it is rather hard for bare nanocrystals to self-assemble into 2D, and especially into 3D, ordered structures. So far, most well-ordered assemblies of nanocrystals have been prepared through a surface modification approach. Efforts have been made to prepare superstructures composed of ZnO nanocrystals but it is rather challenging to obtain well-ordered 3D ZnO superlattices. Researchers in China have now found that ZnO nanocrystals capped with ionic liquids spontaneously assemble into a three-dimensional lattice. Apparently, simply drying a solution of the modified ZnO nanocrystals is all that is needed for the superlattice to form. The presence of the ionic liquid prevents the nanocrystals from aggregating.
Zinc Oxide (ZnO) has long been used in its powdered form as pigments in paints, coatings for papers, in the commercial manufacture of rubber goods as well as UVA and UVB blocker and mild antimicrobial in cosmetics. ZnO is also one of the most important semiconductor compounds and numerous reports have been documented in the literature about the preparation and characterization of ZnO nanocrystals. While polycrystalline forms of ZnO have been used for technical uses such as piezoelectric transducers, light emitting diodes, and transparent conducting films, the progress in developing single crystal bulk ZnO have brought its promise as a wide band gap semiconductor to the fore. Superstructures formed from ZnO nanocrystal quantum dots may find applications in various areas such as optics, electronics and magnetism. For these 2D and 3D superstructures to be useful they need to be well-ordered. Usually, nanocrystals without any surface modification are less stable and they usually undergo aggregation or crystal growth, and consequently it is rather hard for bare nanocrystals to self-assemble into 2D, and especially into 3D, ordered structures. So far, most well-ordered assemblies of nanocrystals have been prepared through a surface modification approach. Efforts have been made to prepare superstructures composed of ZnO nanocrystals but it is rather challenging to obtain well-ordered 3D ZnO superlattices. Researchers in China have now found that ZnO nanocrystals capped with ionic liquids spontaneously assemble into a three-dimensional lattice. Apparently, simply drying a solution of the modified ZnO nanocrystals is all that is needed for the superlattice to form. The presence of the ionic liquid prevents the nanocrystals from aggregating.
 Subscribe to our Nanotechnology Spotlight feed
Subscribe to our Nanotechnology Spotlight feed





