Showing Spotlights 489 - 496 of 559 in category All (newest first):
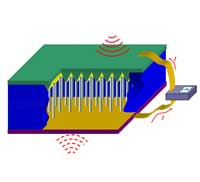 For the over 100 million people worldwide who suffer from diabetes, testing blood glucose is the only way to be sure that it is within normal range and allows them to adjust the insulin dose if it is not. The current method for monitoring blood glucose requires poking your finger to obtain a blood sample. The equipment needed to perform the blood test includes a needle device for drawing blood, a blood glucose meter, single-use test strips, and a log book. Now imagine this scenario: your doctor implants a tiny device the size of a rice grain under your skin. This device automatically and accurately measures your blood glucose levels at whatever intervals, even constantly if required. It transmits the data to an external transceiver. If any abnormality is detected, the device warns you and automatically transmits the data to your doctor's computer. This scenario is one of the many promises of nanomedicine where in-situ, real-time and implantable biosensing, biomedical monitoring and biodetection will become an everyday fact of normal healthcare. Nanosensors are already under intense development in laboratories around the world. One of the important components for implantable nanosensors is an independent power source, either a nanobattery or a nanogenerator that harvests energy from its environment, so that the sensor can operate autonomously. Not only has such a nanogenerator now been developed, but a new prototype has been demonstrated to effectively generate electricity inside biofluid, e.g. blood. This is an important step towards self-powered nanosystems.
For the over 100 million people worldwide who suffer from diabetes, testing blood glucose is the only way to be sure that it is within normal range and allows them to adjust the insulin dose if it is not. The current method for monitoring blood glucose requires poking your finger to obtain a blood sample. The equipment needed to perform the blood test includes a needle device for drawing blood, a blood glucose meter, single-use test strips, and a log book. Now imagine this scenario: your doctor implants a tiny device the size of a rice grain under your skin. This device automatically and accurately measures your blood glucose levels at whatever intervals, even constantly if required. It transmits the data to an external transceiver. If any abnormality is detected, the device warns you and automatically transmits the data to your doctor's computer. This scenario is one of the many promises of nanomedicine where in-situ, real-time and implantable biosensing, biomedical monitoring and biodetection will become an everyday fact of normal healthcare. Nanosensors are already under intense development in laboratories around the world. One of the important components for implantable nanosensors is an independent power source, either a nanobattery or a nanogenerator that harvests energy from its environment, so that the sensor can operate autonomously. Not only has such a nanogenerator now been developed, but a new prototype has been demonstrated to effectively generate electricity inside biofluid, e.g. blood. This is an important step towards self-powered nanosystems.
Jul 6th, 2007
 If you have seen the movie The Matrix then you are familiar with 'jacking in' - a brain-machine neural interface that connects a human brain to a computer network. For the time being, this is still a sci-fi scenario, but don't think that researchers are not heavily working on it. What is already reality today is something called neuroprosthetics, an area of neuroscience that uses artificial microdevices to replace the function of impaired nervous systems or sensory organs. Different biomedical devices implanted in the central nervous system, so-called neural interfaces, already have been developed to control motor disorders or to translate willful brain processes into specific actions by the control of external devices. These implants could help increase the independence of people with disabilities by allowing them to control various devices with their thoughts (not surprisingly, the other candidate for early adoption of this technology is the military). The potential of nanotechnology application in neuroscience is widely accepted. Especially single-walled carbon nanotubes (SWCNT) have received great attention because of their unique physical and chemical features, which allow the development of devices with outstanding electrical properties. In a crucial step towards a new generation of future neuroprosthetic devices, a group of European scientists developed a SWCNT/neuron hybrid system and demonstrated that carbon nanotubes can directly stimulate brain circuit activity.
If you have seen the movie The Matrix then you are familiar with 'jacking in' - a brain-machine neural interface that connects a human brain to a computer network. For the time being, this is still a sci-fi scenario, but don't think that researchers are not heavily working on it. What is already reality today is something called neuroprosthetics, an area of neuroscience that uses artificial microdevices to replace the function of impaired nervous systems or sensory organs. Different biomedical devices implanted in the central nervous system, so-called neural interfaces, already have been developed to control motor disorders or to translate willful brain processes into specific actions by the control of external devices. These implants could help increase the independence of people with disabilities by allowing them to control various devices with their thoughts (not surprisingly, the other candidate for early adoption of this technology is the military). The potential of nanotechnology application in neuroscience is widely accepted. Especially single-walled carbon nanotubes (SWCNT) have received great attention because of their unique physical and chemical features, which allow the development of devices with outstanding electrical properties. In a crucial step towards a new generation of future neuroprosthetic devices, a group of European scientists developed a SWCNT/neuron hybrid system and demonstrated that carbon nanotubes can directly stimulate brain circuit activity.
Jul 5th, 2007
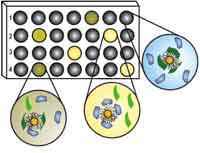 Biomarkers are of increasing importance in modern medicine for the purpose of early detection and diagnosis of a disease, for instance cancer. Biomarkers are mostly protein molecules that can be measured in blood, other body fluids, and tissues to assess the presence or state of a disease. For example, the presence of an antibody may indicate an infection or an antigen, such as PSA, might indicate the presence of prostate-specific cancer cells. Although protein-based approaches to early detection and diagnosis of cancer have a clear advantage over other, more invasive, methods, protein detection is a challenging problem owing to the structural diversity and complexity of the target analytes. State-of-the-art detection methods have limited application due to their high production cost and instability. Another limitation of current proteomic diagnostics is the limitation of arrays to one or a few markers only; in other words, you can only test for the specific markers that you are looking for and not generally measure levels of proteins in your blood in order to detect anomalies. A novel nanotechnology based protein detector array could change that.
Biomarkers are of increasing importance in modern medicine for the purpose of early detection and diagnosis of a disease, for instance cancer. Biomarkers are mostly protein molecules that can be measured in blood, other body fluids, and tissues to assess the presence or state of a disease. For example, the presence of an antibody may indicate an infection or an antigen, such as PSA, might indicate the presence of prostate-specific cancer cells. Although protein-based approaches to early detection and diagnosis of cancer have a clear advantage over other, more invasive, methods, protein detection is a challenging problem owing to the structural diversity and complexity of the target analytes. State-of-the-art detection methods have limited application due to their high production cost and instability. Another limitation of current proteomic diagnostics is the limitation of arrays to one or a few markers only; in other words, you can only test for the specific markers that you are looking for and not generally measure levels of proteins in your blood in order to detect anomalies. A novel nanotechnology based protein detector array could change that.
Jul 2nd, 2007
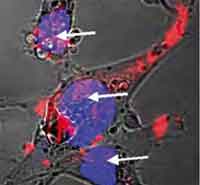 In chemotherapy doctors are using a carpet bombing approach to destroy cancer cells: the patient is pumped full of cytotoxic drugs, that go everywhere in the body, with the hope that enough of the drugs reach the cancer cells and target their nuclear DNA to damage it or destroy the cell. Not only do chemotherapeutic techniques have a range of often serious side effects, mainly affecting all the fast-dividing cells of the body, it also has been shown that often less than 1% of the administered drug molecules enter tumor cells and bind to the nuclear DNA. Another complication is drug resistance of cancer cells. This actually is one of the main causes of failure in the treatment of cancer. Dividing cancer cells acquire genetic changes at a high rate, which means that the cells in a tumor that are resistant to a particular drug will survive and multiply. The result is the re-growth of a tumor that is not sensitive to the original drug. Cancer researchers are looking to nanoparticles as a drug carrier capable of localizing and directly releasing drugs into the cell nucleus, thereby circumventing the multidrug-resistance and intracellular drug-resistance mechanisms to effectively deliver drugs to the vicinity of DNA, leading to a high therapeutic efficacy. Scientists now have developed nanoparticles capable of localizing into the nucleus, giving hope to a much more effective cancer chemotherapy that allows to pinpoint individual cells.
In chemotherapy doctors are using a carpet bombing approach to destroy cancer cells: the patient is pumped full of cytotoxic drugs, that go everywhere in the body, with the hope that enough of the drugs reach the cancer cells and target their nuclear DNA to damage it or destroy the cell. Not only do chemotherapeutic techniques have a range of often serious side effects, mainly affecting all the fast-dividing cells of the body, it also has been shown that often less than 1% of the administered drug molecules enter tumor cells and bind to the nuclear DNA. Another complication is drug resistance of cancer cells. This actually is one of the main causes of failure in the treatment of cancer. Dividing cancer cells acquire genetic changes at a high rate, which means that the cells in a tumor that are resistant to a particular drug will survive and multiply. The result is the re-growth of a tumor that is not sensitive to the original drug. Cancer researchers are looking to nanoparticles as a drug carrier capable of localizing and directly releasing drugs into the cell nucleus, thereby circumventing the multidrug-resistance and intracellular drug-resistance mechanisms to effectively deliver drugs to the vicinity of DNA, leading to a high therapeutic efficacy. Scientists now have developed nanoparticles capable of localizing into the nucleus, giving hope to a much more effective cancer chemotherapy that allows to pinpoint individual cells.
Jun 22nd, 2007
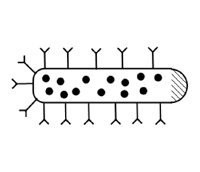 The ideal drug carrier may be something out of science fiction. In principle, it is injected into the body and transports itself to the correct target, such as a tumor, and delivers the required dose at this target. This idealized concept was first proposed by Paul Ehrlich at the beginning of the 20th century and was nicknamed the "magic bullet" concept. With the advent of nanotechnology and nanomedicine this dream is rapidly becoming a reality. Nanotechnology has already been applied to drug delivery and cosmetics through the use of liposomal technology, and now nanoparticles and nanotubes present an exciting and more promising alternative.
The ideal drug carrier may be something out of science fiction. In principle, it is injected into the body and transports itself to the correct target, such as a tumor, and delivers the required dose at this target. This idealized concept was first proposed by Paul Ehrlich at the beginning of the 20th century and was nicknamed the "magic bullet" concept. With the advent of nanotechnology and nanomedicine this dream is rapidly becoming a reality. Nanotechnology has already been applied to drug delivery and cosmetics through the use of liposomal technology, and now nanoparticles and nanotubes present an exciting and more promising alternative.
Jun 19th, 2007
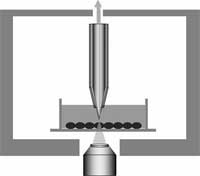 Cells are the smallest 'brick' in life's building structures. Every living organism is made of cells. Individual cells carry their own DNA and have their own life cycle. Considering that larger organisms, such as humans, are basically huge, organized cell cooperatives, the study of individual live cells is a hugely important scientific task. Among the most significant technical challenges for performing successful live-cell imaging experiments is to maintain the cells in a healthy state and functioning normally on the microscope stage while being illuminated. Especially if scientists want to look into cellular processes that occur in cells in their natural state and that cannot be observed by traditional cytological methods. It is well known that cells move, grow, duplicate, and move from point A to point B. Up to now people studied these mechanical properties with optical microscopes because it is the most common and simple method, very efficient, a very well developed and advanced technology. However, with optical microscopes detection is limited to objects no smaller than the wavelengths of the visible region of light, roughly between 400 and 700 nanometers. Distances or movement smaller than this range cannot be seen with these instruments. Researchers in Kyoto, Japan have applied a near-field optical approach to measure cell mechanics and were able to show intriguing data of nanoscale cell membrane dynamics associated with different phenomena of the cell's life, such as cell cycle and cell death.
Cells are the smallest 'brick' in life's building structures. Every living organism is made of cells. Individual cells carry their own DNA and have their own life cycle. Considering that larger organisms, such as humans, are basically huge, organized cell cooperatives, the study of individual live cells is a hugely important scientific task. Among the most significant technical challenges for performing successful live-cell imaging experiments is to maintain the cells in a healthy state and functioning normally on the microscope stage while being illuminated. Especially if scientists want to look into cellular processes that occur in cells in their natural state and that cannot be observed by traditional cytological methods. It is well known that cells move, grow, duplicate, and move from point A to point B. Up to now people studied these mechanical properties with optical microscopes because it is the most common and simple method, very efficient, a very well developed and advanced technology. However, with optical microscopes detection is limited to objects no smaller than the wavelengths of the visible region of light, roughly between 400 and 700 nanometers. Distances or movement smaller than this range cannot be seen with these instruments. Researchers in Kyoto, Japan have applied a near-field optical approach to measure cell mechanics and were able to show intriguing data of nanoscale cell membrane dynamics associated with different phenomena of the cell's life, such as cell cycle and cell death.
Jun 11th, 2007
 In our May 7 spotlight "The potential and the pitfalls of nanomedicine" we took a general look at the potential implications of nanomedicine and addressed some ethical issues that arise as the technology develops. In part two of this article we now take a closer look at emerging nanomedical techniques such as nanosurgery, tissue engineering, nanoparticle-enabled diagnostics, and targeted drug delivery. Again, the ethical issues inherent in these emerging medical technologies need to be considered. There are established principals for ethical assessment of existing, conventional, medical technologies and a new research article examines if and how these principals can be extended to nanomedicine.
In our May 7 spotlight "The potential and the pitfalls of nanomedicine" we took a general look at the potential implications of nanomedicine and addressed some ethical issues that arise as the technology develops. In part two of this article we now take a closer look at emerging nanomedical techniques such as nanosurgery, tissue engineering, nanoparticle-enabled diagnostics, and targeted drug delivery. Again, the ethical issues inherent in these emerging medical technologies need to be considered. There are established principals for ethical assessment of existing, conventional, medical technologies and a new research article examines if and how these principals can be extended to nanomedicine.
May 23rd, 2007
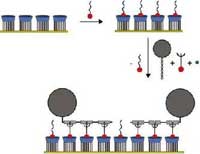 In 2005, researchers in the Netherlands developed the concept of a "molecular printboard" (named for its parallels with a computer motherboard) - a monolayer of host molecules on a solid substrate on which guest molecules can be attached with control over position, binding strength, and binding dynamics. Molecules can be positioned on the printboard using supramolecular contact printing and supramolecular dip-pen nanolithography. In this way, nanoscale patterns can be written and erased on the printboard. This technique, which combines top-down fabrication (lithography) with bottom-up methods (self-assembly), has now been applied to proteins. The resulting "protein printboards", allowing the capture and immobilization of proteins with precise control over specificity, strength and orientation, allows the fabrication of protein chips for applications in proteomics. They will play a major role in unraveling the human protein map, just as special chips were instrumental in mapping human DNA.
In 2005, researchers in the Netherlands developed the concept of a "molecular printboard" (named for its parallels with a computer motherboard) - a monolayer of host molecules on a solid substrate on which guest molecules can be attached with control over position, binding strength, and binding dynamics. Molecules can be positioned on the printboard using supramolecular contact printing and supramolecular dip-pen nanolithography. In this way, nanoscale patterns can be written and erased on the printboard. This technique, which combines top-down fabrication (lithography) with bottom-up methods (self-assembly), has now been applied to proteins. The resulting "protein printboards", allowing the capture and immobilization of proteins with precise control over specificity, strength and orientation, allows the fabrication of protein chips for applications in proteomics. They will play a major role in unraveling the human protein map, just as special chips were instrumental in mapping human DNA.
May 21st, 2007
 For the over 100 million people worldwide who suffer from diabetes, testing blood glucose is the only way to be sure that it is within normal range and allows them to adjust the insulin dose if it is not. The current method for monitoring blood glucose requires poking your finger to obtain a blood sample. The equipment needed to perform the blood test includes a needle device for drawing blood, a blood glucose meter, single-use test strips, and a log book. Now imagine this scenario: your doctor implants a tiny device the size of a rice grain under your skin. This device automatically and accurately measures your blood glucose levels at whatever intervals, even constantly if required. It transmits the data to an external transceiver. If any abnormality is detected, the device warns you and automatically transmits the data to your doctor's computer. This scenario is one of the many promises of nanomedicine where in-situ, real-time and implantable biosensing, biomedical monitoring and biodetection will become an everyday fact of normal healthcare. Nanosensors are already under intense development in laboratories around the world. One of the important components for implantable nanosensors is an independent power source, either a nanobattery or a nanogenerator that harvests energy from its environment, so that the sensor can operate autonomously. Not only has such a nanogenerator now been developed, but a new prototype has been demonstrated to effectively generate electricity inside biofluid, e.g. blood. This is an important step towards self-powered nanosystems.
For the over 100 million people worldwide who suffer from diabetes, testing blood glucose is the only way to be sure that it is within normal range and allows them to adjust the insulin dose if it is not. The current method for monitoring blood glucose requires poking your finger to obtain a blood sample. The equipment needed to perform the blood test includes a needle device for drawing blood, a blood glucose meter, single-use test strips, and a log book. Now imagine this scenario: your doctor implants a tiny device the size of a rice grain under your skin. This device automatically and accurately measures your blood glucose levels at whatever intervals, even constantly if required. It transmits the data to an external transceiver. If any abnormality is detected, the device warns you and automatically transmits the data to your doctor's computer. This scenario is one of the many promises of nanomedicine where in-situ, real-time and implantable biosensing, biomedical monitoring and biodetection will become an everyday fact of normal healthcare. Nanosensors are already under intense development in laboratories around the world. One of the important components for implantable nanosensors is an independent power source, either a nanobattery or a nanogenerator that harvests energy from its environment, so that the sensor can operate autonomously. Not only has such a nanogenerator now been developed, but a new prototype has been demonstrated to effectively generate electricity inside biofluid, e.g. blood. This is an important step towards self-powered nanosystems.
 Subscribe to our Nanotechnology Spotlight feed
Subscribe to our Nanotechnology Spotlight feed





