Showing Spotlights 185 - 192 of 203 in category All (newest first):
 Due to their unique structural and electrical properties, carbon nanotubes have been extensively investigated as promising catalyst supports to improve the efficiency of direct ethanol/methanol fuel cells. CNTs have a significantly higher electronic conductivity and an extremely higher specific surface area in comparison with the most widely-used Vulcan XC-72R carbon support. Several approaches, such as electrochemical reduction, electroless deposition, spontaneous reduction, sonochemical technique, microwave-heated polyol process, and nanoparticle decoration on chemically oxidized nanotube sidewalls, have been reported to form CNT-supported platinum catalysts. Some remarkable progress has been made in synthesis techniques; however, pioneering breakthroughs have not been made yet in terms of cost-effectiveness catalyst activity, durability, and chemical-electrochemical stability. Nanotechnology researchers in the U.S. have now discovered that platinum nanoparticles selectively grow on carbon nanotubes in accordance with single-stranded DNA locations.
Due to their unique structural and electrical properties, carbon nanotubes have been extensively investigated as promising catalyst supports to improve the efficiency of direct ethanol/methanol fuel cells. CNTs have a significantly higher electronic conductivity and an extremely higher specific surface area in comparison with the most widely-used Vulcan XC-72R carbon support. Several approaches, such as electrochemical reduction, electroless deposition, spontaneous reduction, sonochemical technique, microwave-heated polyol process, and nanoparticle decoration on chemically oxidized nanotube sidewalls, have been reported to form CNT-supported platinum catalysts. Some remarkable progress has been made in synthesis techniques; however, pioneering breakthroughs have not been made yet in terms of cost-effectiveness catalyst activity, durability, and chemical-electrochemical stability. Nanotechnology researchers in the U.S. have now discovered that platinum nanoparticles selectively grow on carbon nanotubes in accordance with single-stranded DNA locations.
Oct 28th, 2009
 The batteries that power our everyday devices, from laptop computers, to mobile phones, watches, toys and flashlights, are a major source of pollution. The average household in the Western world uses about 20 batteries a year, resulting in hundreds of thousands of tons of discarded batteries that end up in landfills. When the battery casing corrodes, toxic heavy metals like mercury and cadmium can leak out and pollute soil and ground water. Researchers have been working on non-metal batteries but so far the performance of the used materials has not been good enough for commercial applications. One way to improve the performance of nonmetal-based energy storage devices is to use composite electrode materials of conductive polymers, deposited as thin layers on a suitable large surface area substrate. Researchers have now developed a novel polypyrrole-cellulose composite electrode material that is mechanically robust, lightweight, and flexible.
The batteries that power our everyday devices, from laptop computers, to mobile phones, watches, toys and flashlights, are a major source of pollution. The average household in the Western world uses about 20 batteries a year, resulting in hundreds of thousands of tons of discarded batteries that end up in landfills. When the battery casing corrodes, toxic heavy metals like mercury and cadmium can leak out and pollute soil and ground water. Researchers have been working on non-metal batteries but so far the performance of the used materials has not been good enough for commercial applications. One way to improve the performance of nonmetal-based energy storage devices is to use composite electrode materials of conductive polymers, deposited as thin layers on a suitable large surface area substrate. Researchers have now developed a novel polypyrrole-cellulose composite electrode material that is mechanically robust, lightweight, and flexible.
Sep 16th, 2009
 In today's Spotlight we take a look at a specific example of the challenges researchers face in improving fuel cell technology and the important role that modern laboratory instruments such as electron microscopes play in their work. Fuel cells have gained a lot of attention because they provide a potential solution to our addiction to fossil fuels. Energy production from oil, coal and gas is an extremely polluting, not to mention wasteful, process that consists of heat extraction from fuel by burning it, conversion of that heat to mechanical energy, and transformation of that mechanical energy into electrical energy. In contrast, fuel cells are electrochemical devices that convert a fuel's chemical energy directly to electrical energy with high efficiency and without combustion (although fuel cells operate similar to batteries, an important difference is that batteries store energy, while fuel cells can produce electricity continuously as long as fuel and air are supplied).
In today's Spotlight we take a look at a specific example of the challenges researchers face in improving fuel cell technology and the important role that modern laboratory instruments such as electron microscopes play in their work. Fuel cells have gained a lot of attention because they provide a potential solution to our addiction to fossil fuels. Energy production from oil, coal and gas is an extremely polluting, not to mention wasteful, process that consists of heat extraction from fuel by burning it, conversion of that heat to mechanical energy, and transformation of that mechanical energy into electrical energy. In contrast, fuel cells are electrochemical devices that convert a fuel's chemical energy directly to electrical energy with high efficiency and without combustion (although fuel cells operate similar to batteries, an important difference is that batteries store energy, while fuel cells can produce electricity continuously as long as fuel and air are supplied).
Aug 6th, 2009
 The field of printable electronics is already well established. The biggest limitation to further increasing the functionality of printed electronics devices is energy management, i.e. the space requirement and cost of batteries. Ideally, power and energy storage devices will be integrated into the manufacturing process to be printed at the same time. What is still needed to complement a further deveopment of printed electronics device technology are truly printable charge storage devices that can be easily fabricated using large-scale, solution-based, roll-to-roll processing, while still displaying good electrochemical performance. Only fully printable charge storage devices would allow for full integration into the manufacturing process of printed electronics.
The field of printable electronics is already well established. The biggest limitation to further increasing the functionality of printed electronics devices is energy management, i.e. the space requirement and cost of batteries. Ideally, power and energy storage devices will be integrated into the manufacturing process to be printed at the same time. What is still needed to complement a further deveopment of printed electronics device technology are truly printable charge storage devices that can be easily fabricated using large-scale, solution-based, roll-to-roll processing, while still displaying good electrochemical performance. Only fully printable charge storage devices would allow for full integration into the manufacturing process of printed electronics.
Apr 20th, 2009
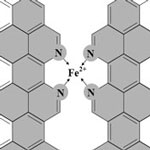 New work by this Canadian research team has now demonstrated that it is possible to significantly increase the catalytic site density of iron-based non-precious metal catalysts (NPMCs) to levels that were not thought possible before. The problem that this work resolves is that of the low activity of NPMCs compared to platinum-based catalysts. The best of these new NPMCs is more than 30 times more active compared to the previous best reported activity for NPMCs, and about 100 times more active than the majority of other NPMCs. Furthermore, their activity has reached about 1/10th the volumetric activity of state-of-the-art platinum-based catalysts (about 50 wt% platinum on carbon), which is the 2010 NPMC activity target set by the U.S. Department of Energy.
New work by this Canadian research team has now demonstrated that it is possible to significantly increase the catalytic site density of iron-based non-precious metal catalysts (NPMCs) to levels that were not thought possible before. The problem that this work resolves is that of the low activity of NPMCs compared to platinum-based catalysts. The best of these new NPMCs is more than 30 times more active compared to the previous best reported activity for NPMCs, and about 100 times more active than the majority of other NPMCs. Furthermore, their activity has reached about 1/10th the volumetric activity of state-of-the-art platinum-based catalysts (about 50 wt% platinum on carbon), which is the 2010 NPMC activity target set by the U.S. Department of Energy.
Apr 8th, 2009
 Platinum nanoparticles are widely used as the cathode material in hydrogen/oxygen fuel cells due to their efficiency in catalyzing the oxygen reduction reaction (ORR), the process that breaks the bonds of the oxygen molecules. Although platinum is still considered the state-of-the-art ORR catalyst, it does not exhibit good stability. Typically, catalyst performance degradation begins as soon as the catalyst is introduced into a fuel cell and continues until it is no longer active. Platinum can lose its effectiveness either by clumping together or by becoming 'poisoned' by carbon monoxide, requiring high hydrogen purity or higher catalyst densities for the fuel cell to stay effective. This, together with the high cost of platinum is seen as one of the major showstoppers to producing mass market fuel cells for commercial applications. Researchers now found that vertically-aligned nitrogen-containing carbon nanotubes could be used as effective ORR electrocatalysts.
Platinum nanoparticles are widely used as the cathode material in hydrogen/oxygen fuel cells due to their efficiency in catalyzing the oxygen reduction reaction (ORR), the process that breaks the bonds of the oxygen molecules. Although platinum is still considered the state-of-the-art ORR catalyst, it does not exhibit good stability. Typically, catalyst performance degradation begins as soon as the catalyst is introduced into a fuel cell and continues until it is no longer active. Platinum can lose its effectiveness either by clumping together or by becoming 'poisoned' by carbon monoxide, requiring high hydrogen purity or higher catalyst densities for the fuel cell to stay effective. This, together with the high cost of platinum is seen as one of the major showstoppers to producing mass market fuel cells for commercial applications. Researchers now found that vertically-aligned nitrogen-containing carbon nanotubes could be used as effective ORR electrocatalysts.
Feb 5th, 2009
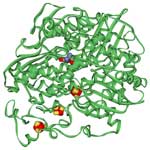 The hydrogen that will power tomorrow's cars is not a naturally occurring resource that can be tapped by drilling a hole in the ground. Hydrogen has to be produced, and that can be done using a variety of resources. The cleanest by far of course would be renewable energy electrolysis: using electricity to split water into hydrogen and oxygen; this electricity could be generated using renewable energy technologies such as wind, solar, geo- and hydrothermal power. As it stands, most of today's hydrogen production is 'dirty' - it is produced from methane in natural gas using high-temperature steam in what is called steam methane reforming. Many research groups around the world are working hard on developing cheap, clean and efficient technologies to produce hydrogen from water, particularly using sunlight (artificial photosynthesis). This would be the ultimate clean, renewable and abundant energy source. However, to become commercially viable, fuel cells have to overcome the barrier of high catalyst cost caused by the exclusive use of expensive platinum and platinum-based catalysts in the fuel-cell electrodes - the reason is that platinum is the most efficient electrocatalyst for accelerating chemical reactions in fuel cells. Scientists have found that platinum catalysts can be replaced with bacteria-produced hydrogenase enzymes that have nickel and iron in their active sites.
The hydrogen that will power tomorrow's cars is not a naturally occurring resource that can be tapped by drilling a hole in the ground. Hydrogen has to be produced, and that can be done using a variety of resources. The cleanest by far of course would be renewable energy electrolysis: using electricity to split water into hydrogen and oxygen; this electricity could be generated using renewable energy technologies such as wind, solar, geo- and hydrothermal power. As it stands, most of today's hydrogen production is 'dirty' - it is produced from methane in natural gas using high-temperature steam in what is called steam methane reforming. Many research groups around the world are working hard on developing cheap, clean and efficient technologies to produce hydrogen from water, particularly using sunlight (artificial photosynthesis). This would be the ultimate clean, renewable and abundant energy source. However, to become commercially viable, fuel cells have to overcome the barrier of high catalyst cost caused by the exclusive use of expensive platinum and platinum-based catalysts in the fuel-cell electrodes - the reason is that platinum is the most efficient electrocatalyst for accelerating chemical reactions in fuel cells. Scientists have found that platinum catalysts can be replaced with bacteria-produced hydrogenase enzymes that have nickel and iron in their active sites.
Dec 10th, 2008
 Modern fuel cells have the potential to revolutionize transportation. Like battery-electric vehicles, fuel cell vehicles are propelled by electric motors. But while battery electric vehicles use electricity from an external source and store it in a battery, fuel cells onboard a vehicle are electrochemical devices that convert a fuel's chemical energy directly to electrical energy with high efficiency and without combustion. One of the leading fuel cell technologies developed, in particular for transportation applications, is the proton exchange membrane (PEM) fuel cell, also known as polymer electrolyte membrane fuel cells - both resulting in the same acronym PEMFC. These fuel cells are powered by the electrochemical oxidation reaction of hydrogen and by the electroreduction of the oxygen contained in air. Presently, platinum-based electrocatalysts are the most widely used in PEM fuel cell prototypes. However, this metal is expensive due to its limited supply and its price is highly volatile. This creates one of the major barriers preventing commercialization of PEMFCs - the lack of suitable materials to make them affordable. Nanotechnology provides solutions to this problem.
Modern fuel cells have the potential to revolutionize transportation. Like battery-electric vehicles, fuel cell vehicles are propelled by electric motors. But while battery electric vehicles use electricity from an external source and store it in a battery, fuel cells onboard a vehicle are electrochemical devices that convert a fuel's chemical energy directly to electrical energy with high efficiency and without combustion. One of the leading fuel cell technologies developed, in particular for transportation applications, is the proton exchange membrane (PEM) fuel cell, also known as polymer electrolyte membrane fuel cells - both resulting in the same acronym PEMFC. These fuel cells are powered by the electrochemical oxidation reaction of hydrogen and by the electroreduction of the oxygen contained in air. Presently, platinum-based electrocatalysts are the most widely used in PEM fuel cell prototypes. However, this metal is expensive due to its limited supply and its price is highly volatile. This creates one of the major barriers preventing commercialization of PEMFCs - the lack of suitable materials to make them affordable. Nanotechnology provides solutions to this problem.
Oct 31st, 2008
 Due to their unique structural and electrical properties, carbon nanotubes have been extensively investigated as promising catalyst supports to improve the efficiency of direct ethanol/methanol fuel cells. CNTs have a significantly higher electronic conductivity and an extremely higher specific surface area in comparison with the most widely-used Vulcan XC-72R carbon support. Several approaches, such as electrochemical reduction, electroless deposition, spontaneous reduction, sonochemical technique, microwave-heated polyol process, and nanoparticle decoration on chemically oxidized nanotube sidewalls, have been reported to form CNT-supported platinum catalysts. Some remarkable progress has been made in synthesis techniques; however, pioneering breakthroughs have not been made yet in terms of cost-effectiveness catalyst activity, durability, and chemical-electrochemical stability. Nanotechnology researchers in the U.S. have now discovered that platinum nanoparticles selectively grow on carbon nanotubes in accordance with single-stranded DNA locations.
Due to their unique structural and electrical properties, carbon nanotubes have been extensively investigated as promising catalyst supports to improve the efficiency of direct ethanol/methanol fuel cells. CNTs have a significantly higher electronic conductivity and an extremely higher specific surface area in comparison with the most widely-used Vulcan XC-72R carbon support. Several approaches, such as electrochemical reduction, electroless deposition, spontaneous reduction, sonochemical technique, microwave-heated polyol process, and nanoparticle decoration on chemically oxidized nanotube sidewalls, have been reported to form CNT-supported platinum catalysts. Some remarkable progress has been made in synthesis techniques; however, pioneering breakthroughs have not been made yet in terms of cost-effectiveness catalyst activity, durability, and chemical-electrochemical stability. Nanotechnology researchers in the U.S. have now discovered that platinum nanoparticles selectively grow on carbon nanotubes in accordance with single-stranded DNA locations.
 Subscribe to our Nanotechnology Spotlight feed
Subscribe to our Nanotechnology Spotlight feed





