Showing Spotlights 89 - 96 of 152 in category All (newest first):
 Silver nanoparticles are one of the most extensively used type of nanoparticles in consumer products due to the unique antibacterial activity of silver. There have been raising environmental concerns over their adverse ecological effects, along with ionic silver potentially released from the particles. To predict the environmental impact of engineered silver nanoparticles, their characterization from environmental matrices should be pursued, yet no field-scale studies are available to date. A new research report was motivated by the fact that silver nanoparticles in consumer products are likely being released during and/or after the product's lifetime. The silver nanoparticles will likely get into wastewater streams and subsequently enter wastewater treatment plants. During wastewater treatment processes, silver nanoparticles may be incorporated into the sewage sludge matrix and concentrated over time.
Silver nanoparticles are one of the most extensively used type of nanoparticles in consumer products due to the unique antibacterial activity of silver. There have been raising environmental concerns over their adverse ecological effects, along with ionic silver potentially released from the particles. To predict the environmental impact of engineered silver nanoparticles, their characterization from environmental matrices should be pursued, yet no field-scale studies are available to date. A new research report was motivated by the fact that silver nanoparticles in consumer products are likely being released during and/or after the product's lifetime. The silver nanoparticles will likely get into wastewater streams and subsequently enter wastewater treatment plants. During wastewater treatment processes, silver nanoparticles may be incorporated into the sewage sludge matrix and concentrated over time.
Oct 4th, 2010
 In nature, uni- and multicellular organisms are capable of reducing and accumulating metal ions as detoxification and homeostasis mechanisms when exposed to metal ion solutions. Although the exact mechanisms and identities of microbial proteins associated for metal nanoparticle synthesis are not clear, two cysteine-rich, heavy metal-binding biomolecules, phytochelatin and metallothionein have been relatively well characterized. Phytochelatins are peptides that are synthesized by the protein phytochelatin synthase and that can form metal complexes with cadmium, copper, silver, lead and mercury, while metallothioneins are gene-encoded proteins capable of directly binding metals such as copper, cadmium, and zinc. This capability of phytochelatin and metallothionein - having different metal binding affinities to various metal ions - has now been employed by researchers for the in vivo biosynthesis of metal nanoparticles by recombinant Escherichia coli.
In nature, uni- and multicellular organisms are capable of reducing and accumulating metal ions as detoxification and homeostasis mechanisms when exposed to metal ion solutions. Although the exact mechanisms and identities of microbial proteins associated for metal nanoparticle synthesis are not clear, two cysteine-rich, heavy metal-binding biomolecules, phytochelatin and metallothionein have been relatively well characterized. Phytochelatins are peptides that are synthesized by the protein phytochelatin synthase and that can form metal complexes with cadmium, copper, silver, lead and mercury, while metallothioneins are gene-encoded proteins capable of directly binding metals such as copper, cadmium, and zinc. This capability of phytochelatin and metallothionein - having different metal binding affinities to various metal ions - has now been employed by researchers for the in vivo biosynthesis of metal nanoparticles by recombinant Escherichia coli.
Sep 24th, 2010
 One possible option for reducing CO2 emissions from power plants is to capture them before they hit the atmosphere and store the gas underground. This technique is called Carbon dioxide Capture and Storage. However, before CO2 can be stored, it must be separated from the other waste gases resulting from combustion or industrial processes. Most current methods used for this type of filtration are expensive and require the use of chemicals. Nanotechnology techniques to fabricate nanoscale thin membranes could lead to new membrane technology that could change that.
Current membranes are in many cases not competitive for large scale applications, because their permeance for carbon dioxide is not high enough. Researchers in Germany have now reported the development and manufacturing of nanometric thin film membranes with record performance.
One possible option for reducing CO2 emissions from power plants is to capture them before they hit the atmosphere and store the gas underground. This technique is called Carbon dioxide Capture and Storage. However, before CO2 can be stored, it must be separated from the other waste gases resulting from combustion or industrial processes. Most current methods used for this type of filtration are expensive and require the use of chemicals. Nanotechnology techniques to fabricate nanoscale thin membranes could lead to new membrane technology that could change that.
Current membranes are in many cases not competitive for large scale applications, because their permeance for carbon dioxide is not high enough. Researchers in Germany have now reported the development and manufacturing of nanometric thin film membranes with record performance.
Sep 21st, 2010
 Along with control of fire, iron smelting is one of the founding technological pillars of civilization. Industry has used the same basic process to make iron for over 3000 years. Yet, it is also one of the major global sources of greenhouse gas release. Iron, a basic commodity, is still produced by the greenhouse gas intensive reduction of iron oxide by carbon-coke and currently accounts for the release of one quarter of worldwide carbon dioxide emissions by industry. For instance, on average 1.9 tonnes of carbon dioxide are emitted for every tonne of steel produced. Due to a large share of coal in the energy mix of current production technology, the CO2 emissions are high. Through a new understanding of the chemistry of iron at high temperature, researchers have uncovered an effective new carbon-dioxide-free process to form iron.
Along with control of fire, iron smelting is one of the founding technological pillars of civilization. Industry has used the same basic process to make iron for over 3000 years. Yet, it is also one of the major global sources of greenhouse gas release. Iron, a basic commodity, is still produced by the greenhouse gas intensive reduction of iron oxide by carbon-coke and currently accounts for the release of one quarter of worldwide carbon dioxide emissions by industry. For instance, on average 1.9 tonnes of carbon dioxide are emitted for every tonne of steel produced. Due to a large share of coal in the energy mix of current production technology, the CO2 emissions are high. Through a new understanding of the chemistry of iron at high temperature, researchers have uncovered an effective new carbon-dioxide-free process to form iron.
Aug 25th, 2010
 Quite a lot of nanotechnology research and manufacturing efforts go into synthesizing metal-based nanoparticles. Unfortunately, some of the nanoparticle manufacturing processes themselves as well as the final nanoparticle materials may be of potential concern for environmental regulators and for researchers attempting to address nanomaterial toxicity. As an alternative to using these potentially hazardous metal-based nanoparticles, some researchers are suggesting the use of naturally occurring nanoparticles. However, this area has not yet been well explored with regard to natural nanoparticles' diverse properties and potential applications. Researchers have now made the discovery that naturally occurring nanoparticles have unique optical properties. In addition, they are less toxic and biodegradable than their synthesized, metal-based counterparts. This discovery makes it likely that scientists will be able to find more biocompatible nanoparticles to replace metal-based nanoparticles, predominantly for biomedical applications.
Quite a lot of nanotechnology research and manufacturing efforts go into synthesizing metal-based nanoparticles. Unfortunately, some of the nanoparticle manufacturing processes themselves as well as the final nanoparticle materials may be of potential concern for environmental regulators and for researchers attempting to address nanomaterial toxicity. As an alternative to using these potentially hazardous metal-based nanoparticles, some researchers are suggesting the use of naturally occurring nanoparticles. However, this area has not yet been well explored with regard to natural nanoparticles' diverse properties and potential applications. Researchers have now made the discovery that naturally occurring nanoparticles have unique optical properties. In addition, they are less toxic and biodegradable than their synthesized, metal-based counterparts. This discovery makes it likely that scientists will be able to find more biocompatible nanoparticles to replace metal-based nanoparticles, predominantly for biomedical applications.
Jul 21st, 2010
 The alarming rise of carbon dioxide in the atmosphere has led a numerous proposals on how to capture and store carbon dioxide in order to mitigate the damaging emissions from fossil fuels. Popular proposals, some already being tested on a large scale, involve carbon sequestration and subsequent storage in geological formations (geo-sequestration). Other ideas revolve around recycling captured carbon dioxide, for instance by converting it into hydrocarbons that can be re-used to make fuel or plastics. While these solutions would result in removing some carbon dioxide from the atmosphere, their disadvantages are that most of them are expensive, technologically challenging, or energy-intensive. Researchers have now presented the first experimental evidence of a new solar conversion process, combining electronic and chemical pathways, for carbon dioxide capture in what could become a revolutionary approach to remove and recycle CO2 from the atmosphere on a large scale. Rather than trying to sequester or hide away excess carbon dioxide, this new method allows it to be stored as solid carbon or converted in useful products.
The alarming rise of carbon dioxide in the atmosphere has led a numerous proposals on how to capture and store carbon dioxide in order to mitigate the damaging emissions from fossil fuels. Popular proposals, some already being tested on a large scale, involve carbon sequestration and subsequent storage in geological formations (geo-sequestration). Other ideas revolve around recycling captured carbon dioxide, for instance by converting it into hydrocarbons that can be re-used to make fuel or plastics. While these solutions would result in removing some carbon dioxide from the atmosphere, their disadvantages are that most of them are expensive, technologically challenging, or energy-intensive. Researchers have now presented the first experimental evidence of a new solar conversion process, combining electronic and chemical pathways, for carbon dioxide capture in what could become a revolutionary approach to remove and recycle CO2 from the atmosphere on a large scale. Rather than trying to sequester or hide away excess carbon dioxide, this new method allows it to be stored as solid carbon or converted in useful products.
Jul 16th, 2010
 Developing chemicals, molecular precursors, and industrial products from petroleum resources is a conventional practice. Plastics, detergents, even pharmaceuticals are derived from petrochemicals. With an increasing focus on the economic and environmental issues associated with the processing of petroleum-based chemicals, scientists are seeking for alternative routes to develop molecules from naturally available plant or crop-based raw materials. Particularly interesting for the fields of nanotechnology is the design and development of soft nanomaterials from renewable sources. Generating these materials from renewable resources could have a significant impact on production technologies and economies.
Developing chemicals, molecular precursors, and industrial products from petroleum resources is a conventional practice. Plastics, detergents, even pharmaceuticals are derived from petrochemicals. With an increasing focus on the economic and environmental issues associated with the processing of petroleum-based chemicals, scientists are seeking for alternative routes to develop molecules from naturally available plant or crop-based raw materials. Particularly interesting for the fields of nanotechnology is the design and development of soft nanomaterials from renewable sources. Generating these materials from renewable resources could have a significant impact on production technologies and economies.
Jun 1st, 2010
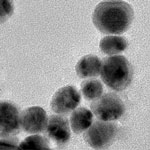 Metal nanomaterials are often synthesized using the toxic reagent formaldehyde at concentrations thousands of times higher than necessary. Many of these same nanomaterials are being investigated for use in cancer treatment - however, there is a risk that they could do more harm than good. The large excess of formaldehyde that is used originates from methods developed 100 years ago. Because these methods work well, they have stood the test of time. By better understanding the role that formaldehyde plays in nanomaterial synthesis it will become possible to reduce or eliminate this toxic reagent. By eliminating formaldehyde it will become safer to prepare these nanomaterials and safer to use them in cancer treatment.
Metal nanomaterials are often synthesized using the toxic reagent formaldehyde at concentrations thousands of times higher than necessary. Many of these same nanomaterials are being investigated for use in cancer treatment - however, there is a risk that they could do more harm than good. The large excess of formaldehyde that is used originates from methods developed 100 years ago. Because these methods work well, they have stood the test of time. By better understanding the role that formaldehyde plays in nanomaterial synthesis it will become possible to reduce or eliminate this toxic reagent. By eliminating formaldehyde it will become safer to prepare these nanomaterials and safer to use them in cancer treatment.
May 24th, 2010
 Silver nanoparticles are one of the most extensively used type of nanoparticles in consumer products due to the unique antibacterial activity of silver. There have been raising environmental concerns over their adverse ecological effects, along with ionic silver potentially released from the particles. To predict the environmental impact of engineered silver nanoparticles, their characterization from environmental matrices should be pursued, yet no field-scale studies are available to date. A new research report was motivated by the fact that silver nanoparticles in consumer products are likely being released during and/or after the product's lifetime. The silver nanoparticles will likely get into wastewater streams and subsequently enter wastewater treatment plants. During wastewater treatment processes, silver nanoparticles may be incorporated into the sewage sludge matrix and concentrated over time.
Silver nanoparticles are one of the most extensively used type of nanoparticles in consumer products due to the unique antibacterial activity of silver. There have been raising environmental concerns over their adverse ecological effects, along with ionic silver potentially released from the particles. To predict the environmental impact of engineered silver nanoparticles, their characterization from environmental matrices should be pursued, yet no field-scale studies are available to date. A new research report was motivated by the fact that silver nanoparticles in consumer products are likely being released during and/or after the product's lifetime. The silver nanoparticles will likely get into wastewater streams and subsequently enter wastewater treatment plants. During wastewater treatment processes, silver nanoparticles may be incorporated into the sewage sludge matrix and concentrated over time.
 Subscribe to our Nanotechnology Spotlight feed
Subscribe to our Nanotechnology Spotlight feed





