| Aug 12, 2024 | |
Self-propelled microrobots enhance industrial chemical reactions |
|
| (Nanowerk Spotlight) Chemical manufacturing has long relied on catalysts - substances that accelerate reactions without being consumed themselves. These catalysts typically exist as static entities, either fixed in place or passively dispersed throughout reaction mixtures. While effective, this approach limits the degree of control chemists have over complex processes. | |
| Enter microrobots - tiny devices ranging from a few micrometers to tens of micrometers in size, about the width of a human hair. These microscopic machines can move autonomously and perform programmed tasks. In recent years, microrobots have shown promise in fields like medicine and environmental cleanup, where they can navigate through bodily fluids or contaminated water to deliver drugs or remove pollutants. | |
| However, applying microrobots to industrial chemistry has proven challenging. Most microrobots developed so far only function in water-based systems, severely limiting their use in chemical manufacturing, which often relies on organic solvents. The materials and propulsion mechanisms that work well in water tend to fail or degrade when exposed to these harsher chemical environments. | |
| Despite these obstacles, the potential benefits of mobile, controllable catalysts have driven researchers to seek solutions. The ability to guide catalysts dynamically through a reaction mixture could enhance efficiency, enable new reaction pathways, and provide unprecedented control over complex chemical processes. | |
| Recent progress in materials science and our understanding of how objects move at the microscale have opened new avenues for tackling these challenges. Researchers have developed more chemically stable nanomaterials and advanced techniques for modifying surfaces at the molecular level. These advancements have expanded the toolkit available for designing microrobots that can withstand and operate in diverse chemical environments. | |
| A team of scientists has now reported a significant breakthrough in this quest. Their work, published in Advanced Functional Materials ("Microrobots Enhancing Synthetic Chemistry Reactions in Non-Aqueous Media"), describes the creation of zeolite-based microrobots capable of catalyzing an important industrial reaction while propelling themselves through an organic solvent. | |
 |
|
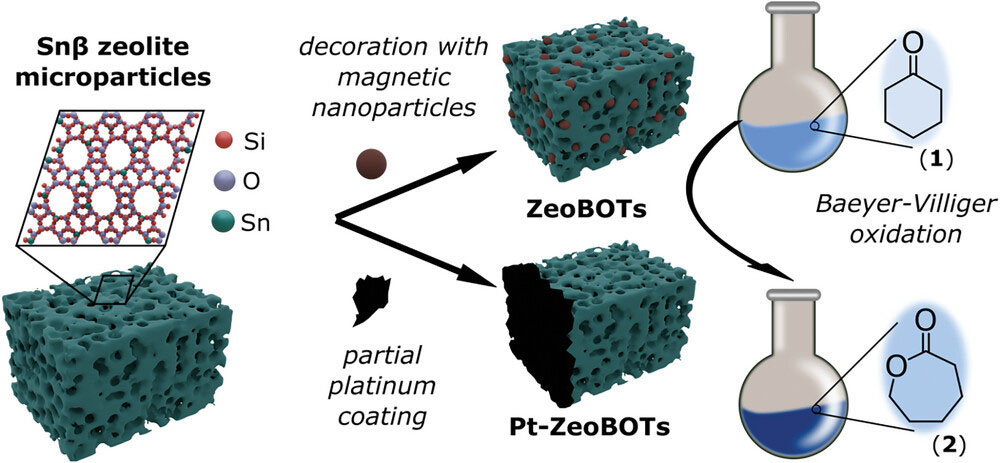
| Design of microrobots by modifying Sn𝛽 zeolite microstructures toward the Baeyer–Villiger oxidation of cyclohexanone (1) to 𝜖-caprolactone (2). (Image: Reproduced from DOI:10.1002/adfm.202409459; CC BY) | |
| Zeolites, a class of porous aluminosilicate materials, have been workhorses of the chemical industry for decades. Known for their stability and versatile catalytic properties, zeolites seemed like ideal candidates for transformation into mobile catalysts. The researchers modified commercially available zeolite particles by replacing aluminum atoms with tin, creating what are known as Sn-beta zeolites. These tin-containing zeolites can catalyze a variety of organic reactions, including the Baeyer-Villiger oxidation - a key process in producing certain pharmaceuticals and other specialized chemicals. | |
| To turn these zeolite particles into self-propelled microrobots, the team explored two strategies. In one approach, they decorated the zeolite surface with magnetic nanoparticles, creating "ZeoBOTs" that could be controlled using external magnetic fields. The second method involved partially coating the zeolites with a thin layer of platinum, resulting in "Pt-ZeoBOTs." In water, platinum can catalyze the breakdown of hydrogen peroxide to produce oxygen bubbles, providing a means of chemical propulsion. | |
| The researchers rigorously characterized their creations using advanced analytical techniques to confirm the successful modification of the zeolite structure and the presence of the added components. A critical test was evaluating how these microrobots performed in non-aqueous environments. The team conducted experiments in pure 1,4-dioxane, an organic solvent commonly used in industrial processes, as well as in mixtures of dioxane and water. | |
| The results revealed important nuances in the microrobots' performance across different solvent conditions. The magnetically-driven ZeoBOTs could be propelled through pure dioxane using a rotating magnetic field, demonstrating their potential for use in fully organic environments. The chemically propelled Pt-ZeoBOTs, however, were unable to move in pure dioxane, likely because the solvent's low polarity prevented the catalytic reaction needed for bubble propulsion. | |
| To understand why solvent conditions affect microrobot propulsion so dramatically, it's helpful to consider the underlying mechanisms. Bubble propulsion, used by the Pt-ZeoBOTs, relies on the catalytic decomposition of hydrogen peroxide into water and oxygen gas. This reaction occurs more readily in polar solvents like water, which can stabilize the charged intermediates formed during the catalytic process. In contrast, non-polar organic solvents like pure dioxane don't support this reaction as effectively, inhibiting bubble formation and, consequently, propulsion. The polarity of a solvent essentially determines how well it can facilitate the chemical reactions necessary for propulsion. | |
| Interestingly, when tested in a 50% dioxane-water mixture, the Pt-ZeoBOTs regained some mobility. This finding suggests that while pure organic solvents pose challenges for certain propulsion mechanisms, mixed solvent systems might offer a workable compromise for some applications. The presence of water in the mixture provides enough polarity to support the catalytic reaction, while the organic component allows for compatibility with a broader range of chemical processes. The researchers noted that this ability to function in mixed solvents could be particularly valuable for reactions that require both organic and aqueous components. | |
| To demonstrate the practical potential of their innovation, the researchers used the ZeoBOTs to catalyze the Baeyer-Villiger oxidation of cyclohexanone to epsilon-caprolactone, an industrially relevant reaction. They compared the performance of mobile ZeoBOTs, propelled by an external magnetic field, to stationary zeolite particles under identical conditions. | |
| The mobile ZeoBOTs significantly outperformed their stationary counterparts. After 5 hours, the magnetically-propelled microrobots achieved a reaction yield of 8% ± 2%, while the static particles managed only 4% ± 1%. This improvement was attributed to the continuous movement of the microrobots through the reaction mixture, which likely enhanced mass transfer and mixing. | |
| The team also investigated how their microrobots behaved on a larger scale, observing their movement and distribution throughout a larger volume of reaction mixture. This analysis revealed an intriguing phenomenon: the microrobots exhibited a degree of coordinated "swarming" behavior. When exposed to the external magnetic field, the ZeoBOTs tended to aggregate and move collectively, forming dynamic patterns within the reaction vessel. | |
| This swarming behavior could have significant implications for the industrial application of microrobots. In principle, it could allow for more efficient coverage of the reaction volume, potentially leading to even greater enhancements in reaction rates and yields. The researchers speculated that by fine-tuning the magnetic field, it might be possible to control these swarms with high precision, directing them to specific regions of a reactor or guiding them through sequential reaction zones in a continuous flow system. | |
| However, the study also identified several challenges that need to be addressed before such microrobots could see widespread industrial adoption. One significant issue was sedimentation - over time, the microrobots tended to settle at the bottom of the reaction vessel, particularly when the magnetic field was applied from below. This sedimentation could potentially limit the long-term effectiveness of the microrobots in industrial-scale reactors. | |
| The researchers proposed several potential solutions to this challenge. One approach could involve periodically reversing the direction of the magnetic field to resuspend settled microrobots. Another possibility is to design microrobots with lower density or altered geometries that resist sedimentation. The team also suggested that continuous flow reactor designs, where the reaction mixture is constantly moving, might help keep the microrobots suspended and active for longer periods. | |
| Another area identified for improvement was the non-uniform propulsion observed among the microrobot population. Some microrobots moved more efficiently than others, likely due to variations in size, shape, or magnetic coating. The researchers suggested that more precise fabrication techniques and stricter quality control measures could help produce a more homogeneous population of microrobots with more consistent performance. | |
| This research represents a significant step toward expanding the application of microrobotics to industrial chemical processes. By successfully adapting zeolites - materials already widely used in the chemical industry - into self-propelled catalysts capable of operating in organic solvents, the researchers have bridged an important gap between the fields of microrobotics and practical chemical manufacturing. | |
| The ability to dynamically control the movement of catalysts through a reaction mixture opens up new possibilities for optimizing chemical processes. It could lead to more efficient use of catalysts, better control over reaction kinetics, and the ability to perform sequential or cascade reactions with greater precision. The magnetic control demonstrated in this study also offers a non-invasive method for manipulating the microrobots, which could be particularly valuable in enclosed or continuous flow reactor systems. | |
| While the current work focused on a single model reaction, the approach could potentially be adapted to a wide range of industrially relevant chemical transformations. The modular nature of the microrobot design, with the zeolite core providing catalytic activity and the magnetic nanoparticles enabling controlled motion, suggests that similar strategies could be applied to other catalyst systems. | |
| This research highlights the potential for microrobotics to transform chemical manufacturing processes. By combining the catalytic properties of well-established industrial materials with the dynamic control offered by microrobotics, it may be possible to develop more efficient, flexible, and sustainable chemical production methods. As this field advances, we may see the emergence of "smart" chemical reactors that use swarms of specialized microrobots to carry out complex multi-step syntheses with unprecedented precision and efficiency. These developments could lead to more sustainable manufacturing processes, accelerated drug discovery, and the ability to create advanced materials with properties tailored at the molecular level. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
