| Aug 09, 2024 | |
Ultrasound-activated nanopatch simplifies nerve repair post-surgery |
|
| (Nanowerk Spotlight) Peripheral nerve injuries pose significant challenges in medical treatment, often leading to long-term disabilities and reduced quality of life. These injuries can result from various causes, including trauma, surgery, and diseases. One particularly common and debilitating form is erectile dysfunction caused by damage to the cavernous nerves during prostate cancer surgery. Despite decades of research, effective treatments for peripheral nerve injuries have remained elusive, largely due to the complexity of nerve regeneration and the limitations of current therapeutic approaches. | |
| Traditional methods for promoting nerve repair have included surgical interventions, pharmacological treatments, and physical therapy. However, these approaches often yield inconsistent results and may not fully restore nerve function. In recent years, electrical stimulation has emerged as a promising strategy for enhancing nerve regeneration. This technique involves applying controlled electrical currents to damaged nerves, which can accelerate axon growth and remyelination. However, conventional electrical stimulation methods typically require invasive electrode implantation and external power sources, limiting their practicality and widespread adoption. | |
| Advances in materials science and nanotechnology have opened up new possibilities for developing innovative nerve repair strategies. Piezoelectric materials, which can generate electrical charges in response to mechanical stress, have garnered particular interest. These materials offer the potential to create self-powered, biocompatible devices that can provide localized electrical stimulation without the need for external power sources or complex implantation procedures. Additionally, progress in tissue engineering has led to the development of scaffolds and hydrogels that can mimic the extracellular matrix and provide a supportive environment for nerve regeneration. | |
| Against this backdrop, researchers have been exploring ways to combine these emerging technologies to create more effective and user-friendly solutions for peripheral nerve repair. A recent study published in Advanced Functional Materials ("An Easy Nanopatch Promotes Peripheral Nerve Repair through Wireless Ultrasound-Electrical Stimulation in a Band-Aid-Like Way") presents a novel approach that integrates piezoelectric materials, conductive hydrogels, and ultrasound stimulation to promote nerve regeneration in a minimally invasive manner. | |
 |
|
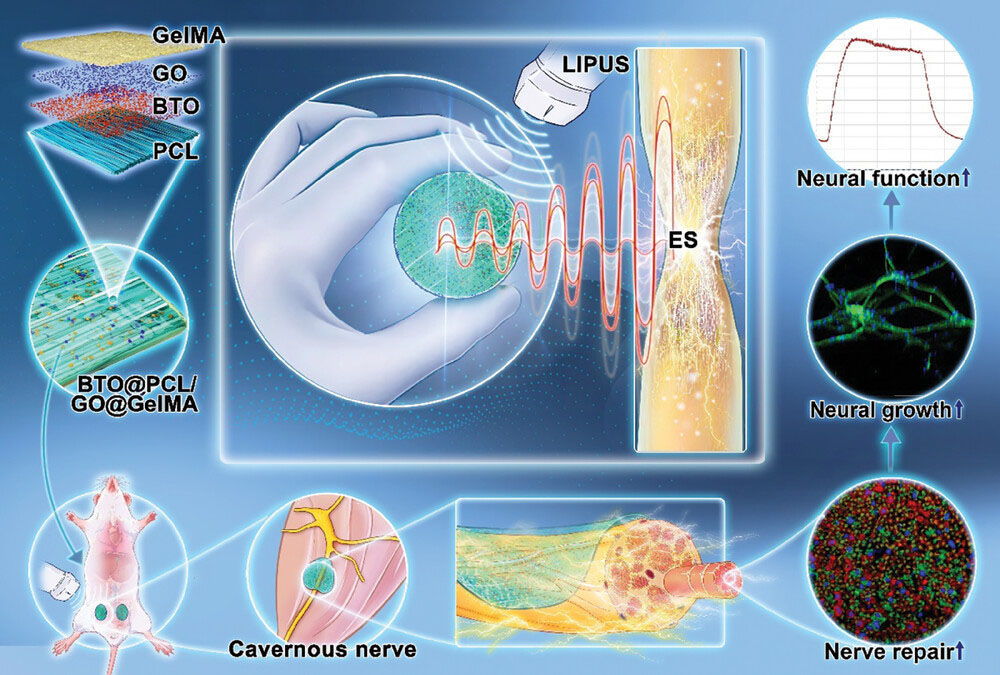
| Self-powered band-aid-type BTO@PCL/GO@GelMA nanopatch for peripheral nerve electrical stimulation repair for biomimetic applications and a schematic diagram of its principle of action. BTO: barium titanate; PCL: polycaprolactone; GO: graphene oxide; GelMA: gelatinmethacryloyl; LIPUS: low-intensity pulsed ultrasound; ES: electrical stimulation. (Image: reproduced with permission by Wiley-VCH Verlag) | |
| The research team, led by scientists from multiple institutions in China, developed a flexible, self-powered nanopatch that can be easily applied to damaged nerves. This "band-aid-like" device consists of two main components: a layer of oriented barium titanate (BTO) nanoparticles incorporated into a polycaprolactone (PCL) nanofiber membrane, and a layer of graphene oxide (GO)-doped gelatin methacryloyl (GelMA) hydrogel. | |
| The BTO@PCL layer serves as the piezoelectric component, capable of generating small electrical currents when subjected to mechanical stress. The GO@GelMA layer provides a conductive and biocompatible interface for interacting with neural tissue. By combining these materials, the researchers created a composite nanopatch that can convert mechanical energy into electrical stimulation while also supporting cell growth and nerve regeneration. | |
| One of the key innovations in this study is the use of low-intensity pulsed ultrasound (LIPUS) to activate the piezoelectric nanopatch. When LIPUS is applied to the area where the nanopatch is attached, it generates mechanical waves that cause the BTO nanoparticles to produce electrical charges. This approach allows for non-invasive, wireless electrical stimulation of the damaged nerves without the need for implanted electrodes or external power sources. | |
| The researchers conducted a series of in vitro and in vivo experiments to evaluate the effectiveness of their nanopatch system. In laboratory tests, they found that the BTO@PCL/GO@GelMA nanopatch promoted the growth and proliferation of Schwann cells, which play a crucial role in peripheral nerve repair. When combined with LIPUS stimulation, the nanopatch significantly enhanced axonal growth compared to control conditions. | |
| To assess the potential clinical applications of their device, the research team tested the nanopatch in a rat model of erectile dysfunction caused by cavernous nerve injury. This condition is a common complication of prostate cancer surgery and serves as a representative example of peripheral nerve damage. The researchers applied the nanopatch to the injured nerves in a manner similar to applying a band-aid and then administered LIPUS treatment over several weeks. | |
| The results of the animal study were promising. Rats treated with the LIPUS-activated nanopatch showed significant improvements in erectile function compared to untreated animals or those receiving only LIPUS or the nanopatch alone. Histological analysis revealed increased smooth muscle content, enhanced endothelial function, and improved nerve regeneration in the treated animals. Importantly, the researchers also observed an increase in the expression of activating transcription factor 3 (ATF3), a protein known to play a key role in nerve regeneration and functional recovery. | |
| The study's findings suggest that this piezoelectric nanopatch system could offer several advantages over existing nerve repair techniques. Its flexible, band-aid-like design allows for easy application to damaged nerves without the need for invasive surgery. The use of LIPUS for activation provides a non-invasive method for delivering electrical stimulation, potentially enabling long-term treatment without the risks associated with implanted electrodes. Additionally, the biocompatible materials used in the nanopatch support cell growth and tissue integration, creating a favorable environment for nerve regeneration. | |
| While the results are encouraging, it is important to note that this research is still at an early stage. Further studies will be needed to optimize the nanopatch design, determine the most effective treatment protocols, and assess long-term safety and efficacy. Additionally, the researchers will need to investigate whether this approach can be applied to other types of peripheral nerve injuries beyond cavernous nerve damage. | |
| This study represents a significant step forward in developing advanced therapies for peripheral nerve repair. By combining piezoelectric materials, conductive hydrogels, and ultrasound stimulation, the researchers have created a novel platform that addresses many limitations of current treatments. The "band-aid-like" design allows for easy application without invasive surgery, while LIPUS activation provides non-invasive electrical stimulation. | |
| If further developed and validated, this technology could offer a more accessible and effective option for patients suffering from various peripheral nerve injuries, including erectile dysfunction after prostate cancer surgery. The potential applications extend beyond this specific condition, possibly encompassing a range of neurological disorders. | |
| However, significant work remains before clinical implementation. Future research must focus on optimizing the nanopatch for human use, determining effective treatment protocols, and conducting extensive safety and efficacy studies. Long-term effects, including the durability of nerve repair and potential side effects, also require investigation. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
