| Aug 07, 2024 | |
Innovative nano scissors enable precise enzyme-free DNA cleavage with light |
|
| (Nanowerk Spotlight) Genetic engineering has brought us revolutionary advances in medicine, biotechnology, and our understanding of life itself. However, the precise manipulation of DNA sequences has remained a formidable challenge. Traditional enzyme-based methods like CRISPR, while powerful, are constrained by their reliance on biological machinery and sensitivity to environmental conditions. These limitations have spurred researchers to explore alternative approaches that could offer greater flexibility and control. | |
| Nanomaterials have emerged as a promising frontier for developing new DNA manipulation techniques. Nanoparticles with unique optical and chemical properties have shown potential for interacting with genetic material in novel ways. Concurrently, advances in laser technology and our understanding of light-matter interactions at the nanoscale have opened up new possibilities for precise, targeted interventions at the molecular level. | |
| One particularly intriguing avenue of research involves the use of reactive oxygen species (ROS) to cleave DNA sequences. This approach has shown promise in photodynamic therapies, where light-activated compounds generate ROS to target specific cells or molecules. Building on this concept, researchers have been exploring how various nanomaterials can be used to generate ROS upon light excitation, potentially offering a new way to manipulate DNA with high precision. | |
| Against this backdrop, a team of researchers from Shenzhen University and collaborating institutions have developed an innovative approach to DNA cleavage that combines several cutting-edge technologies. Their work, published in Laser Photonics Reviews ("Light-Guided Genetic Scissors Based on Phosphorene Quantum Dot"), introduces a system called TADPOLE (Targeted DNA Precision Oriented Laser Excision) that takes advantage of the unique properties of black phosphorus quantum dots (BPQDs) to achieve site-specific DNA cutting with remarkable precision and adaptability. | |
| The TADPOLE system represents a novel leap in DNA cleavage technology by leveraging the unique properties of BPQDs for an enzyme-free approach. Unlike CRISPR, which requires precise biological conditions for enzyme activity, TADPOLE utilizes BPQDs to generate reactive oxygen species (ROS) through multiphoton absorption, enabling precise site-specific DNA cleavage. This innovation not only broadens the range of potential applications by functioning across diverse environmental conditions but also enhances the feasibility of in vivo applications with its use of lower-energy light. TADPOLE's departure from traditional enzyme-based methods opens new possibilities for genetic engineering, presenting a versatile tool that overcomes the limitations of current technologies. | |
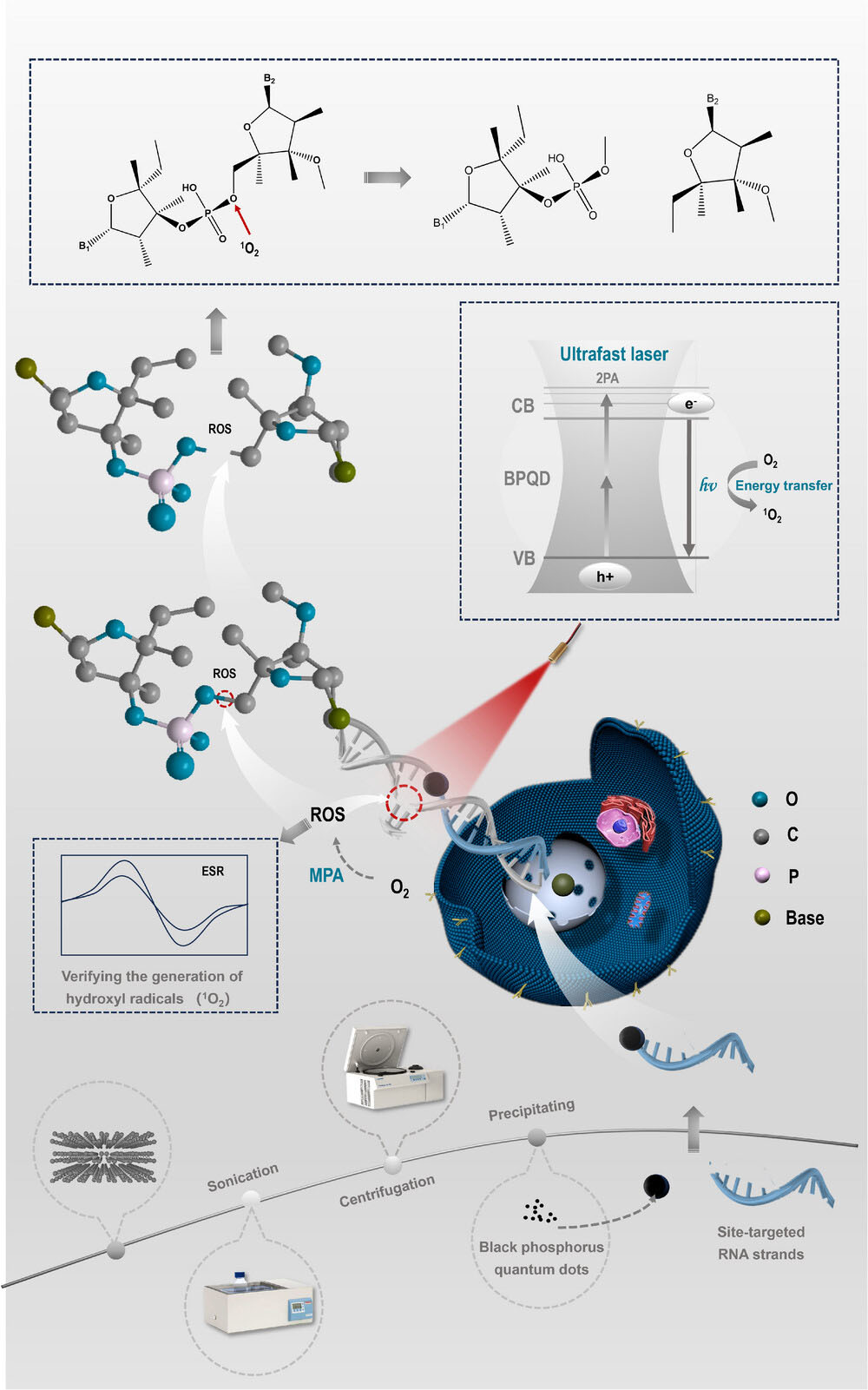 |
|
| Schematic illustration of the study. The process begins with the fabrication of BPQDs from bulk Black Phosphorus (BP) powder, followed by their characterization and confirmation of multiphoton absorption (MPA) properties through the Z-scan technique. The BPQDs are then assessed for their ability to generate reactive peroxy species (1O2-) using Electron Spin Resonance (ESR) spectroscopy. Subsequently, silver (Ag) is integrated into the BPQDs, forming a complex with SH-RNA strands that resemble a tadpole structure. The RNA strand ("tail") enables site-selective DNA sequence binding, while the BPQD ("head") generates hydroxyl radicals through multiphoton absorption when exposed to an 800 nm laser. This results in the creation of the TADPOLE system, which provides an efficient, enzyme-independent, and site-selective gene cleaving mechanism. (Image: Reproduced with permission by Wiley-VCH Verlag) | |
| Black phosphorus, a material that has garnered significant attention in recent years, possesses exceptional electronic and optical properties. When scaled down to quantum dot size, it exhibits even more intriguing characteristics. The researchers chose BPQDs for their system due to their ability to interact with light in complex ways, allowing for precise control over energy absorption and emission. | |
| The TADPOLE system consists of BPQDs decorated with silver atoms and conjugated to guide RNA sequences. This "tadpole-like" structure allows for targeted binding to specific DNA sequences. When irradiated with an ultrafast laser, the BPQDs generate ROS through a process called multiphoton absorption. These localized ROS then cleave the DNA at the targeted site. | |
| What sets TADPOLE apart from existing methods is its combination of high specificity, environmental resilience, and the ability to use lower-energy light for activation. The researchers demonstrated that TADPOLE can maintain high activity across a much broader range of temperatures, salt concentrations, and pH levels compared to CRISPR-based systems. This robustness could make TADPOLE a valuable tool for applications where precise control of reaction conditions is challenging. | |
| Dr. Changle Meng, co-first author of the study, explains the key principles driving their research: "This study aims to overcome the limitations of conventional DNA cleavage approaches by introducing complementary RNA sequences to guide the cleavage site. The precision of DNA cleavage is grounded in complementary base pairing principles, offering a controlled method compared to relying solely on nanoparticle structure." | |
| Meng further elaborates: "Our innovation leverages the efficiency of black phosphorus in generating reactive oxygen species (ROS), enhancing ROS production capabilities in our system. Importantly, we utilize the multi-absorption properties of black phosphorus quantum dots to concentrate strong ROS, primarily singlet oxygen, within a defined range." | |
| Dr. Zhi Chen, another co-first author, adds insight on the practical application: "This approach enables highly efficient, site-specific cleavage within a restricted site while avoiding unintended DNA sequences. Here, we demonstrate the functionality of a DNA strand ('tail')-guided black phosphorus quantum dot ('head') system, accurately capturing the reverse-complementary DNA strand in any sequence, with cleavage triggered by an 800 nm laser." | |
| A key innovation in TADPOLE is the use of complementary RNA sequences to guide the cleavage site. This approach offers more precise control over where DNA cutting occurs compared to relying solely on the structure of nanoparticles. By combining this targeting mechanism with the efficient ROS generation capabilities of BPQDs, the researchers created a system that can achieve highly specific DNA cleavage while minimizing damage to unintended sequences. | |
| The team conducted a series of experiments to characterize the BPQD nanoparticles and verify their ability to generate ROS upon laser irradiation. They used various advanced microscopy and spectroscopy techniques to analyze the structure and composition of the nanoparticles. Importantly, they confirmed that the BPQDs could absorb multiple photons of light simultaneously, allowing them to be activated by longer-wavelength light that can penetrate deeper into biological tissues. | |
| To demonstrate the DNA cleavage capability of TADPOLE, the researchers performed both gel electrophoresis and fluorescence-based assays. They showed that the system could selectively cut DNA at targeted sites, with minimal off-target effects. The specificity was further validated using DNA templates with intentionally mismatched sequences, where TADPOLE showed significantly reduced activity. | |
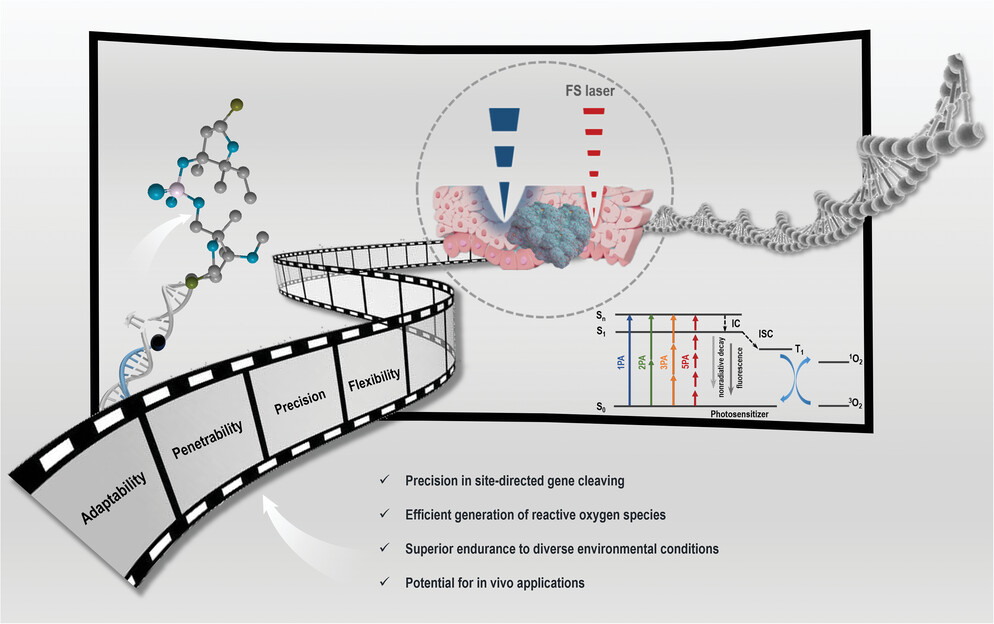 |
|
| Advantages and prospects of TADPOLE. (Image: Reproduced with permission by Wiley-VCH Verlag) | |
| One of the most intriguing aspects of TADPOLE is its potential for in vivo applications. The use of near-infrared light for excitation, coupled with the precise localization of ROS generation, could allow for targeted gene editing within living organisms with minimal collateral damage. To explore this possibility, the team conducted experiments in human cancer cell lines, specifically MCF-7 and MDA-MB-231 cells. | |
| In these experiments, the researchers introduced TADPOLE components labeled with fluorescent markers into the cells. They then used confocal microscopy to observe what happened when the cells were exposed to laser light. Remarkably, they saw that DNA cleavage occurred only in cells containing the correctly matched TADPOLE components, and only when those cells were irradiated with light. This demonstrates that TADPOLE can function with high specificity even in the complex environment of a living cell. | |
| The researchers also compared TADPOLE's performance to CRISPR across various environmental conditions. TADPOLE maintained high activity over a much broader range of temperatures (2-47 °C) compared to CRISPR (37-47 °C). It also showed superior tolerance to variations in salt concentrations and pH levels. This environmental resilience could make TADPOLE particularly useful in scenarios where maintaining strict reaction conditions is impractical. | |
| While the results are promising, the researchers acknowledge that further work is needed to optimize the system for broader applications. Challenges include improving the scalability of TADPOLE production and conducting more extensive biological validation studies. The potential long-term effects of introducing nanoparticles into biological systems also warrant careful investigation. | |
| “The development of TADPOLE represents a significant step forward in the field of enzyme-free DNA manipulation,” concludes Prof. Han Zhang, who led this work.” By combining insights from materials science, photonics, and molecular biology, the researchers have created a versatile tool that could expand the possibilities for genetic engineering. The system's ability to operate under a wide range of conditions, coupled with its use of lower-energy light for activation, opens up new avenues for in vivo gene editing and other applications where traditional methods face limitations.” | |
| Looking ahead, the researchers believe that TADPOLE and similar technologies could have far-reaching implications for clinical medicine and genetic engineering. The ability to precisely manipulate DNA in living cells, without the constraints of traditional enzyme-based methods, could lead to new therapeutic approaches for genetic disorders, more effective cancer treatments, and advanced tools for studying gene function. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
