| Jul 31, 2024 | |
Automated DNA nanotube synthesis method advances molecular engineering capabilities |
|
| (Nanowerk Spotlight) DNA nanotechnology has emerged as a powerful tool for manipulating matter at the nanoscale, offering unprecedented control over the positioning and assembly of molecular components. This field has seen remarkable progress since its inception in the 1980s, with researchers developing increasingly sophisticated methods to create complex structures using DNA as a building material. The programmable nature of DNA, with its predictable base-pairing rules, has allowed scientists to design intricate architectures ranging from simple geometric shapes to functional nanomachines. | |
| Despite these advances, the synthesis of DNA nanostructures has remained a largely manual and time-consuming process. Traditional approaches often require the precise mixing of hundreds of unique DNA strands, followed by careful temperature control to guide self-assembly. This laborious methodology has limited the scalability and widespread adoption of DNA nanotechnology, particularly for applications requiring rapid prototyping or large-scale production. | |
| The challenges in automating DNA nanostructure synthesis stem from the delicate balance required in the assembly process. Unlike the well-established automated methods for synthesizing linear DNA or peptide sequences, creating three-dimensional DNA structures involves complex spatial arrangements and multiple concurrent interactions. Previous attempts to streamline this process have been hindered by the need for extensive human intervention and the difficulty in monitoring and optimizing assembly in real-time. | |
| Recent developments in microfluidics, single-molecule fluorescence microscopy, and computational algorithms have opened new avenues for addressing these long-standing challenges. Advances in precision fluid handling have enabled better control over reaction conditions, while improvements in imaging technologies have made it possible to observe molecular interactions at unprecedented resolution. Concurrently, the rise of machine learning and automated data analysis has provided tools to rapidly process and interpret the vast amounts of information generated during nanostructure assembly. | |
| These technological convergences have set the stage for a potential revolution in DNA nanotechnology, promising to transform the field from a specialized art to a more accessible and scalable science. The ability to automate both the synthesis and characterization of DNA nanostructures could dramatically accelerate the design-build-test cycle, enabling faster iteration and optimization of novel architectures. | |
| In this context, a team of researchers led by Gonzalo Cosa and Hanadi F. Sleiman at McGill University has developed a pioneering automated method for synthesizing and characterizing DNA nanostructures. Their work, published in Advanced Materials ("Automated Synthesis of DNA Nanostructures"), represents a significant step towards realizing the full potential of DNA nanotechnology by addressing the long-standing challenge of automating the assembly process. | |
 |
|
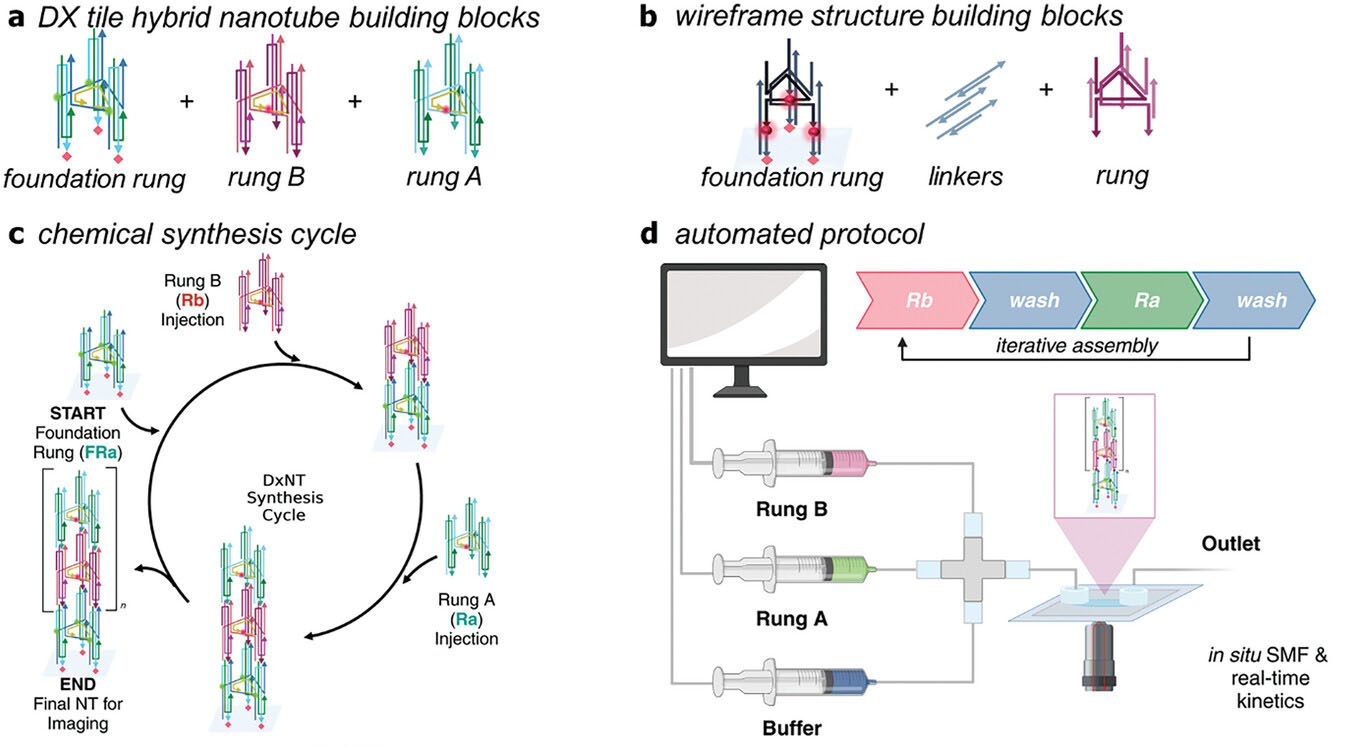
| Synthesis protocols were followed for two structures, a) a DX tile—wireframe hybrid DNA nanotube design (DxNT) where each cycle adds two types of rungs (A and B); and b) a flexible DNA wireframe design, adding a set of three linkers and one rung per cycle. c) Synthesis cycle to construct a DX tile—wireframe DNA nanotube design (DxNT) one unit at a time. The foundation rung (FRa) is attached to the surface; rungs (Ra, Rb) are sequentially added during each cycle. The number of cycles dictates the final size and length of the structure. d) Schematic of prototype DNA nanotube synthesizer. Rungs (A and B) for DxNT, or rungs and linkers for the flexible DNA wireframe nanotube and buffer, are automatically pumped onto the glass coverslip in a time-programmed sequence, facilitating control over length and sequence in the final assembly. (Image: Adapted from DOI:10.1002/adma.202403477, CC BY) | |
| The researchers' approach centers on a custom-built "DNA nanoassembler" that combines microfluidics, single-molecule fluorescence imaging, and automated data analysis to create and evaluate DNA nanotubes with precise control over their length and sequence. This system allows for the stepwise addition of DNA building blocks, or "rungs," to construct nanotubes directly on a glass surface. | |
| One of the key innovations in this work is the use of a simplified set of DNA components. Rather than relying on hundreds of unique DNA strands, the team designed a modular system using just a few types of DNA "rungs" that can be assembled in various combinations. This approach not only simplifies the synthesis process but also allows for greater flexibility in designing different nanotube structures. | |
| The automated synthesis begins with the attachment of a "foundation rung" to the glass surface. The nanoassembler then sequentially adds additional rungs according to a programmed sequence. Each step in the assembly process is monitored in real-time using single-molecule fluorescence microscopy, allowing the researchers to observe the growth of individual nanotubes and assess the efficiency of each addition step. | |
| To optimize the assembly process, the team conducted detailed kinetic studies, measuring the rate at which different rungs were added to the growing nanotubes. These experiments revealed interesting dynamics, such as faster assembly rates for rungs added further away from the glass surface. This information was used to fine-tune the synthesis parameters, including flow rates and incubation times, to maximize efficiency and yield. | |
| A critical component of the automated system is its ability to rapidly analyze the assembled structures. The researchers developed a machine learning algorithm based on K-means clustering to automatically count the number of fluorescent labels in each nanotube. This allowed for quick assessment of the structural integrity and completeness of the synthesized nanotubes without the need for time-consuming manual analysis. | |
| The versatility of the automated system was demonstrated by synthesizing two different types of DNA nanostructures: rigid DX-tile-based nanotubes and flexible wireframe structures. This flexibility showcases the potential of the method to create a wide range of DNA-based architectures for various applications. | |
| One particularly innovative aspect of the system is its ability to selectively detach completed nanotubes from the surface. By incorporating specific DNA sequences that can be triggered to release the nanotubes, the researchers created a method to harvest the finished structures while simultaneously preparing the surface for another round of synthesis. This feature has the potential to significantly increase the production capacity of the system. | |
| The implications of this automated synthesis method extend far beyond the specific nanotubes created in this study. By drastically reducing the time and expertise required to create complex DNA nanostructures, this technology could accelerate research and development across a wide range of fields. Potential applications include the creation of nanoscale sensors for medical diagnostics, the development of drug delivery systems with precise targeting capabilities, and the fabrication of nanoscale electronic components. | |
| Moreover, the ability to rapidly iterate and test different nanotube designs could lead to the discovery of novel structures with unexpected properties or functions. The automated nature of the system also opens the door to high-throughput experimentation, potentially allowing researchers to explore a much larger design space than was previously feasible. | |
| While the current system is limited in its production scale, suitable primarily for single-molecule assays, it represents a significant step towards larger-scale production of DNA nanostructures. The principles and methods developed in this work could be scaled up or adapted to other types of DNA architectures, potentially leading to industrial-scale production of DNA-based nanomaterials. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
