| Jul 23, 2024 | |
Nanoscale high-entropy liquid metal alloys promise advancements in catalysis and materials science |
|
| (Nanowerk Spotlight) Liquid metals have long fascinated materials scientists due to their unique properties that bridge the gap between solid and liquid states of matter. These materials, which remain fluid at or near room temperature, offer a wealth of potential applications across various scientific domains. | |
| In recent years, researchers have been particularly interested in exploring liquid metals as supports for catalysts, where one element is incorporated into the liquid metal matrix at the atomic level or as small clusters. This configuration allows access to the catalytic performance of high-melting-point metals in a pseudo-liquid state at near-room temperatures, using only minute quantities of the active material. | |
| Despite these advances, liquid metal alloys and liquid metal-supported catalysts have typically been limited to systems containing only a few elements. This limitation has prevented them from reaching the realm of high-entropy alloys (HEAs), which are known for their superior mechanical and thermal properties in the solid state. HEAs are characterized by their inherently disordered crystal structures and high concentration of lattice defects, opening up a vast and relatively unexplored compositional space for catalytic and reactive applications. | |
| The concept of high-entropy alloys has gained significant traction in materials science due to the synergistic effects of their multi-elemental constituents, often referred to as the "cocktail effect". These effects are typically achieved through the elemental dispersion of the added constituents. While solid HEAs have been extensively studied for catalysis, focusing on defect engineering and strategies for surface and oxide layer doping, the potential of liquid metal solvents as a dynamic platform for developing multi-elemental and high-entropy liquid alloy systems has remained largely unexplored. | |
| In a recent study published in the journal Small Structures ("Atomic Dispersion via High-Entropy Liquid Metal Alloys"), researchers in Australia have made significant strides in this direction by synthesizing high-entropy liquid metal alloys (HELMAs) at the nanoscale. This innovative approach leverages the distinctive characteristics of gallium-based alloys, which exhibit an exceptional potential to dissolve and reconfigure a wide array of elements within the liquid metal matrix. | |
 |
|
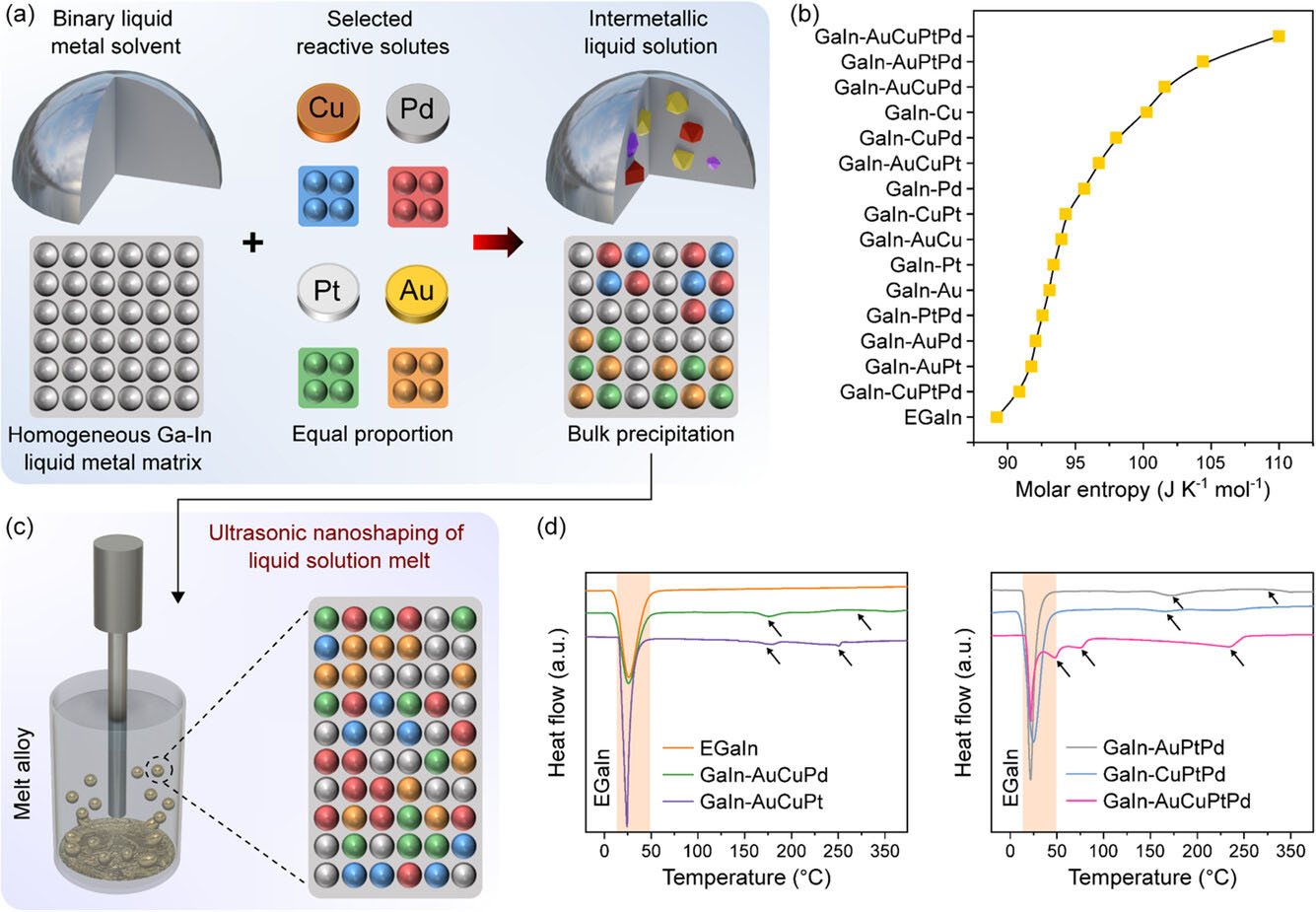
| Schematic representation of the synthesis of High-Entropy Liquid Metal Alloys and their thermal analysis. a) Alloying procedure for making HELMAs from equal proportions of reactive elements in a liquid metal matrix and a representation of the possible precipitation reactions of intermetallic compounds in the liquid metallic solution. b) Entropy calculations of selected combination of multicomponent liquid metals and HELMAs. c) Procedure for the fabrication of nanoscale HELMAs via an ultrasonication method and representation of the high-entropy single solution melt. d) Differential scanning calorimetry analysis (DSC) of the HELMAs with arrows highlighting the phase transition events. (Image: Reproduced from DOI:10.1002/sstr.202400294, CC BY) | |
| The research team developed a method to create HELMAs by dissolving an equiatomic mixture of gold (Au), copper (Cu), platinum (Pt), and palladium (Pd) into a gallium (Ga) and indium (In) based eutectic liquid metal solvent. This process resulted in multielemental high-entropy liquid metal solutions with unique properties. | |
| One of the key advantages of these nanoscale HELMAs is their ability to solvate multiple metallic elements at room temperature while promoting their atomic dispersion at elevated concentrations. The researchers found that the entropy estimations for HELMAs surpass those of high-temperature molten metals, leading to the realization of high-entropy liquid metal systems at room temperature. | |
| To demonstrate the potential of these HELMAs in enhancing the activities of nanocatalysts, the team conducted a proof-of-concept comparison using the hydrogen evolution reaction (HER). In this experiment, they observed atomic dispersion of Pt in a senary GaIn-AuCuPtPd HELMA, contrasting with lower entropy systems in which Pt forms discernible clusters. This finding suggests that HELMAs could lead to catalytic systems with enhanced and tailored activities. | |
| The synthesis process for these HELMAs involved a low-impact, two-step method. First, the reactive solute elements (Au, Cu, Pt, and Pd) were thermally dissolved into the EGaIn liquid metal base at 550°C for 5 hours, forming a homogeneous liquid metal melt with high configurational entropy. In the second step, HELMA nanoparticles were generated via sonication, performed at 250°C in a thermally controlled dispersion medium for 30 minutes. This method preserved the high-entropy characteristics of the melt and avoided undesired phase segregation within the nanoparticles. | |
| The resulting HELMA nanodroplets exhibited a complex structure, comprising a high-entropy metallic core encapsulated within a high-entropy oxide surface layer. Transmission electron microscopy coupled with energy-dispersive X-ray spectroscopy (TEM/EDX) revealed a uniform elemental distribution throughout the nanoscale droplets, highlighting the effectiveness of sonication as an accessible tool for realizing balanced elemental dispersion in liquid high-entropy alloy systems at the nanoscale. | |
| Further analysis using electron energy loss spectroscopy (EELS) and near-edge X-ray absorption fine structure (NEXAFS) provided insights into the electronic structure and local bonding environment of the HELMA nanodroplets. These techniques revealed a multi-tiered structure marked by surface plasmon resonance, an intermediary state, and a bulk plasmon state, emphasizing the complex mixed liquid-solid microstructure induced by the high entropy. | |
| The researchers also investigated the catalytic performance of the HELMA nanodroplets for the hydrogen evolution reaction at room temperature. They found that the GaIn-AuCuPtPd electrode, which includes all active elements and presents the highest entropy, displayed the highest activity with the lowest HER overpotential. Importantly, the HELMA nanodroplets demonstrated high stability in a test involving 3000 cycles, with only a slight increase in overpotential observed after cycling. | |
| This work represents a significant step forward in the field of liquid metal alloys and high-entropy materials. By creating nanoscale high-entropy liquid metal alloys that incorporate noble metals under mild conditions, the researchers have opened up new possibilities for tailoring catalytic systems with enhanced activities. The ability to achieve atomic dispersion of elements like platinum within these high-entropy configurations could lead to more efficient and effective catalysts for a wide range of applications. | |
| Moreover, the high-entropy characteristics of the HELMA nanodroplets effectively limit multiphasic segregation of solid and intermetallic species at high elemental concentrations, addressing a common challenge in the development of multi-component catalysts. The competing solvation phenomena observed within the nanoscale liquid metal matrix of the HELMA nanodroplets could be leveraged for selective atomic dispersion of metallic elements, offering a new approach to catalyst design. | |
| The implications of this research extend beyond noble metals. The liquid metal solvents used in this study open up the possibility of incorporating a wide range of elements, including earth-abundant elements and more exotic materials such as reactive rare earth elements. This versatility provides opportunities for the customization of purpose-built high-entropy liquid metal-based systems utilizing the full spectrum of the periodic table. | |
| As research in this field progresses, we can anticipate the development of new catalysts with unprecedented activities and selectivities, potentially revolutionizing various industrial processes and energy technologies. While the synthesis of these high-entropy liquid metal systems requires elevated temperatures, the resulting materials remain liquid at room temperature. This property could potentially open up new avenues for materials processing and manufacturing at lower temperatures than traditional solid alloys, which might lead to more energy-efficient and sustainable production methods for certain applications. | |
| This study marks a significant advance in the field of liquid metal alloys and high-entropy materials. By demonstrating the synthesis of nanoscale high-entropy liquid metal alloys with atomic dispersion of noble metals, the researchers have opened new avenues for catalyst design and materials engineering. As research in this field progresses, we may see innovations in industrial catalysis, energy technologies, and materials processing. While much work remains to fully realize the potential of these materials, this study lays a solid foundation for future explorations in catalysis, materials science, and nanotechnology. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
