| Jul 18, 2024 | |
Defect-engineered nanoparticles enable precise tracking and delivery of drugs in cells |
|
| (Nanowerk Spotlight) The field of drug delivery has long been challenged by the need for precise, targeted methods to transport therapeutic agents within the body. Traditional approaches often struggle with issues like premature drug release, poor cellular uptake, and inadequate tracking of drug carriers. These limitations have spurred researchers to explore innovative materials and technologies that can overcome these hurdles. | |
| One promising avenue has been the development of nanoscale metal-organic frameworks (nanoMOFs) - highly porous, crystalline structures composed of metal ions linked by organic molecules. Their unique properties, including large surface areas and tunable structures, have made them attractive candidates for drug delivery applications. | |
| However, despite their potential, the use of nanoMOFs in drug delivery has been hampered by several factors. Chief among these has been the difficulty in understanding how these particles interact with cells and move through the body. Without this crucial knowledge, optimizing nanoMOFs for effective and safe drug delivery has remained challenging. Additionally, creating nanoMOFs that can be easily tracked within cells while maintaining their drug-carrying capacity has proven elusive. | |
| Recent advances in imaging technologies, particularly in the realm of single-particle tracking, have opened new possibilities for studying nanoparticle behavior in biological systems. Concurrently, progress in materials science has allowed for more precise control over nanoMOF structures and properties. These developments have set the stage for a deeper investigation into how nanoMOFs can be engineered and utilized for drug delivery. | |
| In this context, a team of researchers at the University of Copenhagen has made significant strides in addressing these longstanding challenges. Their work, published in Advanced Materials ("Defect-Engineered Metal–Organic Frameworks as Nanocarriers for Pharmacotherapy: Insights into Intracellular Dynamics at The Single Particle Level"), presents a novel approach to creating and studying nanoMOFs for drug delivery applications. The researchers have developed a method to create "defect-engineered" nanoMOFs - particles with intentionally introduced imperfections that can be exploited for improved functionality. | |
 |
|
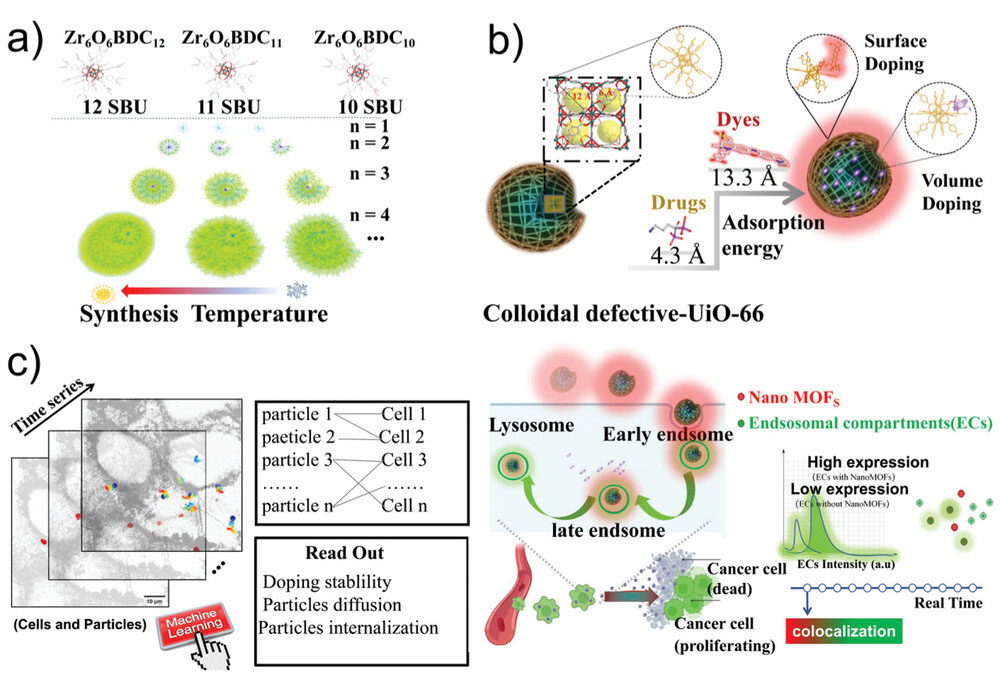
| Representation of the methodology development of nanoMOFs as drug delivery vehicles. a) Cartoon representation of missing linker’s dynamic growth in UiO-66 by 12, 11, and 10 branches of a tree diagram. b) Schematic diagram of surface modification and volume modification. c) Representation of the framework for parallelized tracking of cell entry pathway of nanoMOFs in HeLa cells aided by machine learning analysis (left); Proposed cell uptake mechanism of ATTO-UiO-66@Al based on the tracking data and the algorithm for colocalization percentage between endo/lysosomal compartments and nanoMOFs (right). (Image: Reproduced from DOI:10.1002/adma.202405898, CC BY) (click on image to enlarge) | |
| The team focused on a specific type of nanoMOF called UiO-66, which is known for its stability and potential in biomedical applications. By carefully controlling the synthesis conditions, they were able to create UiO-66 particles with a high density of structural defects. These defects serve a dual purpose: they increase the particle's capacity to carry drugs and provide sites for attaching fluorescent molecules that allow the particles to be tracked within cells. | |
| The researchers used a fluorescent dye called ATTO 655 to label the nanoMOFs. This dye was chosen for its stability and its ability to emit light at wavelengths that are easily distinguishable from the natural fluorescence of cellular components. By attaching ATTO 655 to the defect sites on the UiO-66 particles, the team created nanoMOFs that could be reliably tracked over extended periods. | |
| To demonstrate the potential of these engineered nanoMOFs for drug delivery, the researchers loaded them with a model drug called Alendronate Sodium (AL), which is used to treat bone disorders. They then conducted a series of experiments to study how these drug-loaded, fluorescent nanoMOFs interacted with cells. | |
| One of the most innovative aspects of this study was the use of advanced imaging and analysis techniques to track individual nanoMOF particles in real-time within living cells. The researchers employed spinning disk confocal microscopy, a high-speed imaging method that allows for the observation of fast-moving particles in three dimensions. They combined this with sophisticated machine learning algorithms to analyze the vast amount of data generated by tracking thousands of particles simultaneously. | |
| This approach allowed the team to observe, for the first time, the detailed journey of nanoMOFs as they entered cells and moved through various cellular compartments. They were able to quantify how many particles were internalized by cells over time and determine the specific pathways by which the nanoMOFs were taken up and processed. | |
| The researchers found that their engineered nanoMOFs were efficiently internalized by cells and followed a specific route through cellular structures called endosomes and lysosomes. These are compartments within cells that are involved in processing and breaking down external materials. By tracking the nanoMOFs through these compartments, the team gained insights into how the particles might release their drug cargo within cells. | |
| To further understand the potential of these nanoMOFs for drug delivery, the researchers conducted experiments to assess how effectively they could release the loaded drug under different conditions. They found that the drug release was slightly faster in acidic conditions, which mimics the environment found in certain cellular compartments and tumor tissues. This property could be advantageous for targeted drug delivery to cancer cells. | |
| The team also evaluated the safety and efficacy of their drug-loaded nanoMOFs. They found that empty nanoMOFs (without drug) showed no toxic effects on healthy cells, even at high concentrations. When loaded with the drug AL, the nanoMOFs demonstrated selective toxicity towards cancer cells while sparing healthy cells. This suggests that the nanoMOFs could potentially improve the therapeutic index of drugs by delivering them more specifically to target cells. | |
| The significance of this work lies in its multifaceted approach to advancing nanoMOF-based drug delivery. By engineering particles with specific defects, the researchers have created a versatile platform that combines drug-carrying capacity with trackability. The use of cutting-edge imaging and analysis techniques has provided unprecedented insights into how these particles behave within cells, addressing a critical knowledge gap in the field. | |
| Moreover, the methods developed in this study could be applied to other types of nanoparticles and drug delivery systems, potentially accelerating progress in the broader field of nanomedicine. The ability to observe and quantify the behavior of individual nanoparticles in living cells opens up new possibilities for optimizing drug delivery vehicles and understanding their interactions with biological systems. | |
| While this research represents a significant step forward, it also highlights areas for future investigation. For instance, studying how these nanoMOFs behave in more complex biological environments, such as animal models, will be crucial for translating this technology towards clinical applications. Additionally, exploring ways to further tune the drug release properties of these particles could lead to even more precise and effective drug delivery systems. | |
| This work exemplifies the power of interdisciplinary research in tackling complex challenges in medicine and materials science. By combining advances in nanomaterial engineering, advanced microscopy, and machine learning, the researchers have provided new tools and insights that could accelerate the development of more effective and safer nanomedicine approaches. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
