| Jun 17, 2024 | |
Engineers develop highly efficient atmospheric water harvester with record-breaking clean water production |
|
| (Nanowerk Spotlight) Water scarcity is a growing global challenge, affecting two-thirds of the world's population to varying degrees. While atmospheric water harvesting has long been considered a potential solution, practical applications have been hindered by slow sorption kinetics (the rate at which water is absorbed), salt leakage, and inefficient water collection methods. | |
| Now, a team of researchers at National University of Singapore has developed a novel atmospheric water harvesting system that addresses these limitations, paving the way for large-scale, efficient water collection from air. | |
| The key to this breakthrough lies in the combination of a high-performance hygroscopic hydrogel and a 3D-printed nanoporous silica substrate. Hygroscopic materials (able to attract and hold water molecules from the surrounding environment), such as metal-organic frameworks and salt-hydrogel composites, have shown promise in capturing water from air due to their high sorption capacity. However, these materials have faced challenges in terms of slow water uptake, salt leakage, and the need for energy-intensive desorption processes to release the captured water. | |
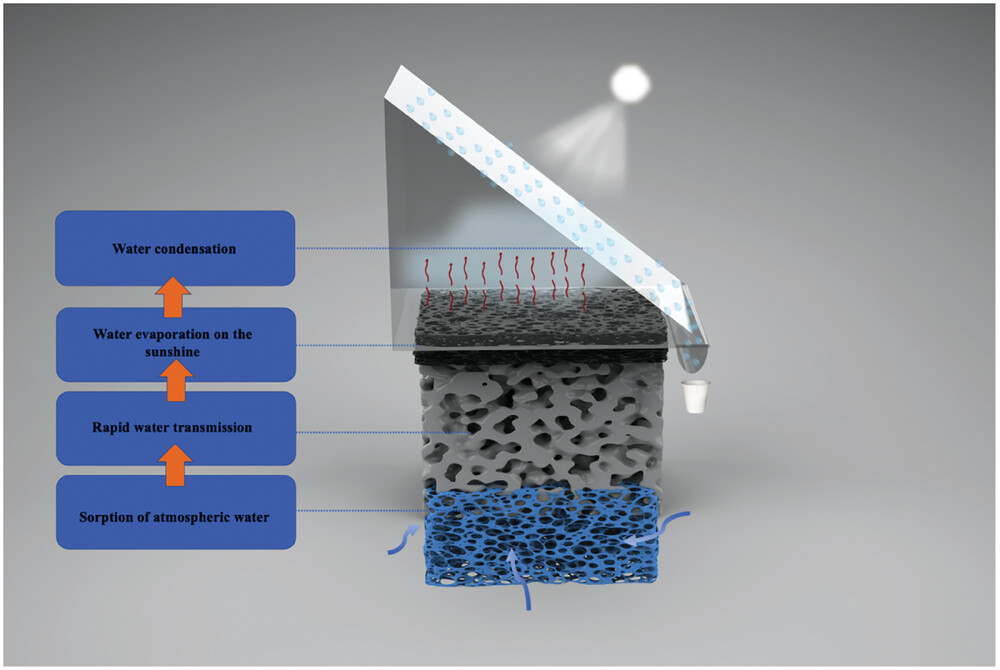 |
|
| Schematic diagram of continuous air water harvesting. From the diagram, it can be seen that when water is sorbed from the air by the hydrogel, it is rapidly transported through microchannels in the nanoporous silica to the evaporation layer. Then, under sunlight, efficient evaporation occurs in the glass chamber. Finally, the water vapor condenses on the surface of hydrophobic glass and the clean water is collected through a trough. (Image: Reproduced from DOI:10.1002/adfm.202402671, CC BY) | |
| In this new study (Advanced Functional Materials, "Nanoporous Silica Lattice Coated with LiCl@PHEA for Continuous Water Harvesting from Atmospheric Humidity"), researchers have synthesized a unique lithium chloride-poly(hydroxyethyl acrylate) (LiCl@PHEA) hydrogel via a one-step UV polymerization process in saturated LiCl solutions. | |
| This approach allows for a high salt content of up to 90 wt% without leakage, resulting in a hydrogel with remarkable air distension ability, expanding over 60 times its original size at 90% relative humidity. The LiCl@PHEA hydrogel exhibits a water sorption efficiency of 11.18 g/g in just 30 minutes, significantly outperforming conventional hygroscopic materials. | |
| To facilitate continuous water collection, the researchers integrated the LiCl@PHEA hydrogel with a 3D-printed nanoporous silica substrate. This substrate features an intricate gyroid structure that enables rapid water transmission from the hydrogel to a porous evaporation layer. | |
| The evaporation layer, coated with a mixture of graphene oxide and carbon black, achieves an extraordinarily high evaporation rate of over 11 kg/m2/h under sunlight, significantly higher than conventional methods. | |
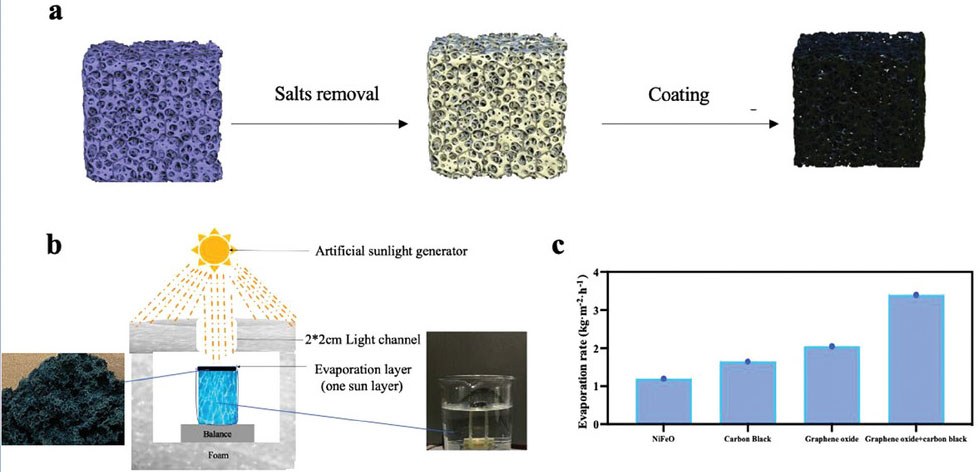 |
|
| Synthesis and performance of the evaporation layer. a) Schematic diagram of the preparation of the evaporation layer. b) Measurement process of the evaporation layer. This ensures that our measurements strictly adhere to solar standards. An enlarged image of the physical layer reveals its highly porous structure. c) Comparison of four different types of coatings: NiFeO, carbon black, graphene, and a mixture of graphene and carbon black. (Image: Reproduced from DOI:10.1002/adfm.202402671, CC BY) | |
| The entire system is housed in a customized 3D-printed shell with a sloped design, allowing for efficient water condensation and collection. As air enters the device from the bottom, the hygroscopic hydrogel captures the moisture and transfers it to the silica substrate. The water then rises through the silica's microchannels to the evaporation layer, where it evaporates and condenses on a hydrophobic surface. The condensed water droplets flow into a collection port, yielding clean water with salt concentrations below 10 ppm. | |
| The prototype device, with a surface area of just 300 cm2, achieves a record-breaking water collection rate of over 5 kg/m2/h, enabling the production of more than 1 liter of clean water per day. This remarkable performance is attributed to the optimized water content in the evaporation layer, which balances the rates of water sorption, transmission, and evaporation. | |
| The implications of this research extend beyond addressing water scarcity. The team has demonstrated the potential for using the collected water in continuous hydrogen production through electrolysis. By connecting the atmospheric water harvester to an electrolysis cell equipped with 3D-printed electrodes, they have created a self-sustaining system for clean hydrogen generation without the need for an external water source. | |
| This groundbreaking work represents a significant step forward in the field of atmospheric water harvesting. The combination of high-performance materials, efficient water transmission, and optimized evaporation conditions has resulted in a device that can continuously collect clean water from air at an unprecedented rate. As the technology is scaled up, it holds the potential to provide a sustainable source of clean water in regions affected by water scarcity, while also enabling decentralized hydrogen production for clean energy applications. | |
| The researchers envision their atmospheric water harvester being deployed in a wide range of settings, from remote communities to urban areas, providing a reliable source of clean water without the need for extensive infrastructure. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
