| Jun 12, 2024 | |
Tiny robot enables unlocks secrets of the gut microbiome, one sample at a time |
|
| (Nanowerk Spotlight) The complex community of microorganisms inhabiting the human digestive tract, known as the gut microbiome, plays a vital role in health and disease. In recent years, research into the gut microbiome has intensified as scientists have uncovered its far-reaching influence on everything from digestive disorders to mental health. However, studying this diverse microbial ecosystem has proven challenging due to limitations in sampling methods. | |
| Traditionally, researchers have relied on stool samples to analyze the gut microbiome. While non-invasive and easy to collect, stool samples primarily reflect the microbiota of the colon and provide minimal insight into the composition of the small intestine. Invasive sampling methods like endoscopy offer a more comprehensive view but come with risks, discomfort, and the potential to alter the very microbiome being studied. | |
| The uneven distribution of microbes throughout the gastrointestinal (GI) tract has emerged as a key factor in understanding the complex interplay between the gut microbiome and various diseases. Studies have shown that the composition of the gut microbiota can vary significantly depending on location, with these differences potentially influencing the effectiveness of treatments. For example, the common inflammatory bowel disease medication mesalamine is less effective in the upper GI tract compared to the lower regions. | |
| To fully grasp the dynamics of the gut microbiome and its role in health and disease, researchers need the ability to study its composition at multiple points along the entire length of the digestive system. Longitudinal analysis, tracking changes over time, and biogeography, mapping microbial populations across different regions, have become crucial tools in this endeavor. However, existing sampling methods have struggled to meet the demands of these approaches. | |
| In a significant step forward, a team of researchers has developed a novel solution: a multiple-sampling capsule robot (MSCR) capable of collecting gut microbiome samples from various locations within the gastrointestinal tract while minimizing cross-contamination. This innovative device, described in a recent paper in Advanced Intelligent Systems ("Multiple Sampling Capsule Robot for Studying Gut Microbiome"), promises to revolutionize the field of gut microbiome research by enabling precise, longitudinal analysis and detailed biogeographical mapping. | |
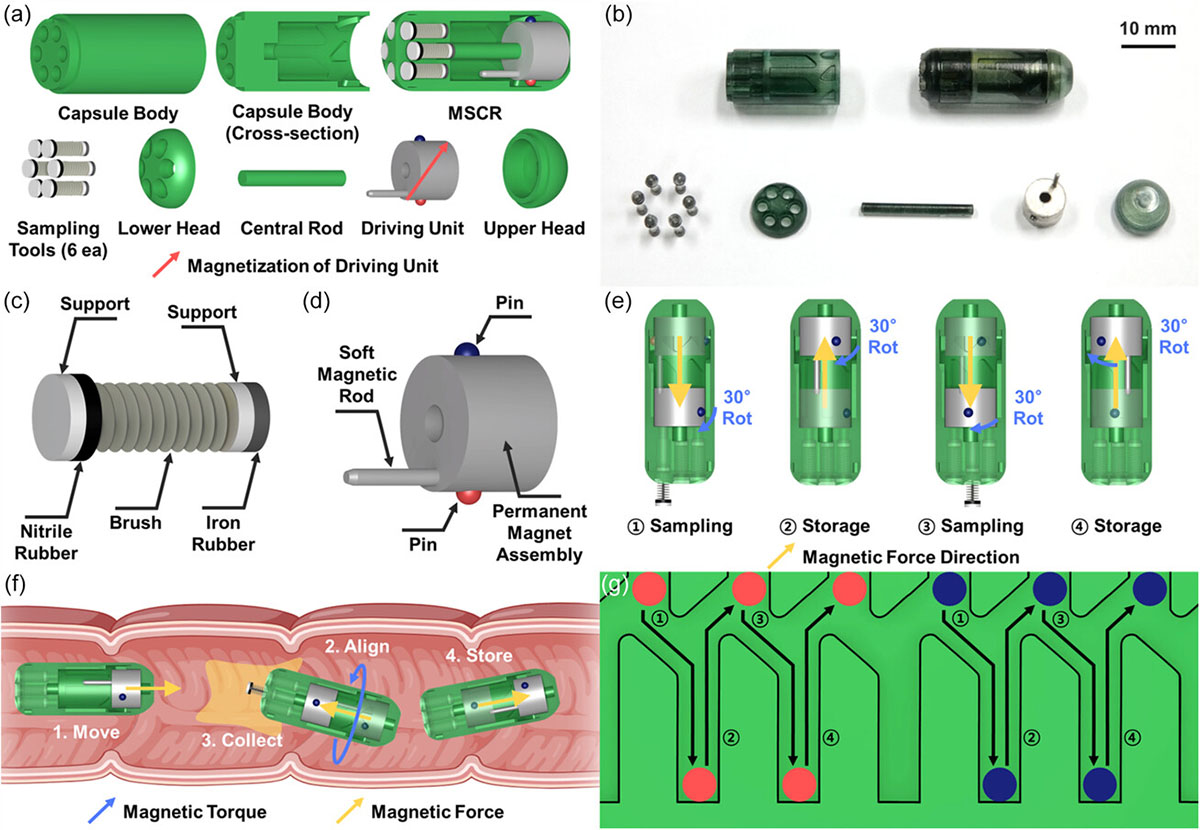
| The MSCR is a small, swallowable capsule measuring 11 mm in diameter and 26 mm in length, ensuring smooth passage through the digestive system. What sets this device apart is its ability to collect up to six separate microbiome samples as it travels through the gut. The capsule features a clever design with six distinct channels, each housing a sampling tool that can be deployed and retracted using an external magnetic field. | |
| By manipulating the magnetic field, researchers can control the orientation and position of the MSCR, aligning the desired sampling channel with the intestinal wall. The sampling tool, which includes a brush for collection and rubber seals to prevent contamination, can then be extended to gather a microbiome sample. Once the sample is collected, the tool retracts back into the capsule, sealing off the channel to avoid cross-contamination with subsequent samples or external intestinal fluids. | |
 |
|
| a) Overall design of the MSCR and its components. b) Fabricated components and fully assembled MSCR. c,d) Schematic designs of the sampling tool and driving unit. e) Working principle of the multiple-sampling mechanism. f ) Sampling process. g) Open view of the inside of the capsule body. It shows the internal groove structure and depicts the path that the two pins follow during the reciprocating motion of the driving unit in the multiple-sampling process. (Reproduced from DOI:10.1002/aisy.202300625, CC BY) (click on image to enlarge) | |
| The ability to collect multiple, uncontaminated samples from specific locations sets the MSCR apart from previous attempts at ingestible sampling devices. Many earlier designs relied on passive movement and physiological factors like pH changes to trigger sampling, limiting their precision and capacity for multiple collections. The MSCR's active locomotion and magnetically controlled sampling mechanisms overcome these limitations. | |
| In basic performance tests, the capsule exhibited impressive precision in both propulsion direction (0.76 ± 0.52°) and channel alignment (0.84 ± 0.55°). Phantom tests, using colored solutions and microbial cultures to simulate the gut environment, confirmed the device's ability to gather multiple samples (averaging 10.3 ± 2.4 mg per sample) with minimal cross-contamination (0.6 ± 0.4%). Ex vivo experiments, conducted using segments of porcine stomach, small intestine, and colon, further validated the MSCR's performance, with an average sample collection of 9.9 ± 1.7 mg. | |
| Notably, 16S rRNA (ribosomal RNA) sequencing analysis of the collected samples closely matched the microbial composition of control samples obtained directly from each organ, confirming the device's sampling accuracy. | |
| Perhaps most exciting are the results of in vivo testing in a porcine model. Guided by a movable external electromagnet system, the MSCR successfully maneuvered within a living pig's stomach, collecting six separate samples. When equipped with a camera module during in vivo testing, the MSCR successfully captured images of the stomach environment, highlighting the potential for integrating imaging capabilities in future iterations of the device. | |
| The development of the MSCR marks a significant milestone in gut microbiome research. By enabling precise, longitudinal sampling and detailed biogeographical mapping of the microbiota across the entire gastrointestinal tract, this innovative device opens up new avenues for understanding the complex relationships between gut microbes, health, and disease. | |
| The MSCR's ability to collect samples from specific locations along the GI tract at multiple time points opens up new possibilities for longitudinal studies. By enabling researchers to track changes in gut microbiome composition over time in response to factors like diet, medication, and disease progression, the MSCR could provide invaluable insights into the dynamics of the gut microbiome and its role in health and disease. | |
| Simultaneously, the ability to map microbial communities across different regions of the gut promises to deepen our understanding of how location-specific variations contribute to gastrointestinal disorders and treatment responses. | |
| As research using the MSCR expands, it has the potential to revolutionize our approach to diagnosing and treating a wide range of conditions linked to the gut microbiome. From inflammatory bowel disease and obesity to autism and Alzheimer's, a more comprehensive understanding of the gut microbial landscape could lead to personalized, targeted therapies and improved patient outcomes. | |
| While further refinements, such as miniaturization, integration of wireless localization, and optimized sample storage, will enhance the MSCR's capabilities, its development represents a groundbreaking step forward in gut microbiome research. As this technology evolves and becomes more widely adopted, it promises to unlock new insights into the intricate world of the gut microbiome, ultimately paving the way for a new era of personalized medicine tailored to the unique microbial profile of each individual. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
