| Jun 07, 2024 | |
New cathode structure boosts stability and performance of sodium-ion batteries |
|
| (Nanowerk Spotlight) Lithium-ion batteries, with their high energy density and long lifetimes, have dominated the rechargeable battery market for decades. However, lithium's scarcity and rising cost have spurred an intense search for alternative battery chemistries using more abundant materials. | |
| Sodium, right below lithium on the periodic table, has emerged as a leading contender. With chemical properties similar to lithium but far greater availability, sodium could enable low-cost rechargeable batteries for large-scale energy storage if researchers can overcome the technical challenges. While the larger size of sodium ions makes it harder for them to squeeze in and out of electrodes, recent breakthroughs in sodium ion conductors and high-capacity electrodes are steadily narrowing the performance gap with lithium-ion batteries. | |
| Sodium superionic conductors (NASICONs) have shown particular promise as cathodes due to their open crystal structure that allows rapid sodium ion diffusion. NASICONs containing vanadium, which can give up multiple electrons per ion, can achieve capacities approaching those of lithium cathodes. However, vanadium's relatively high cost and toxicity have motivated efforts to partially replace it with cheaper, more benign elements like iron and manganese. | |
| Now, a team led by Assistant Professor Edison H. Ang at Nanyang Technological University in Singapore has developed an innovative NASICON cathode that substitutes nearly half the vanadium with iron while boosting performance. As reported in the journal Advanced Science ("Pearl-Structure-Enhanced NASICON Cathode toward Ultrastable Sodium-Ion Batteries"), their Na3.05V1.03Fe0.97(PO4)3 (NVFP) cathode incorporates a conductive carbon framework that improves conductivity and structural stability, enabling ultra-stable cycling at high rates. | |
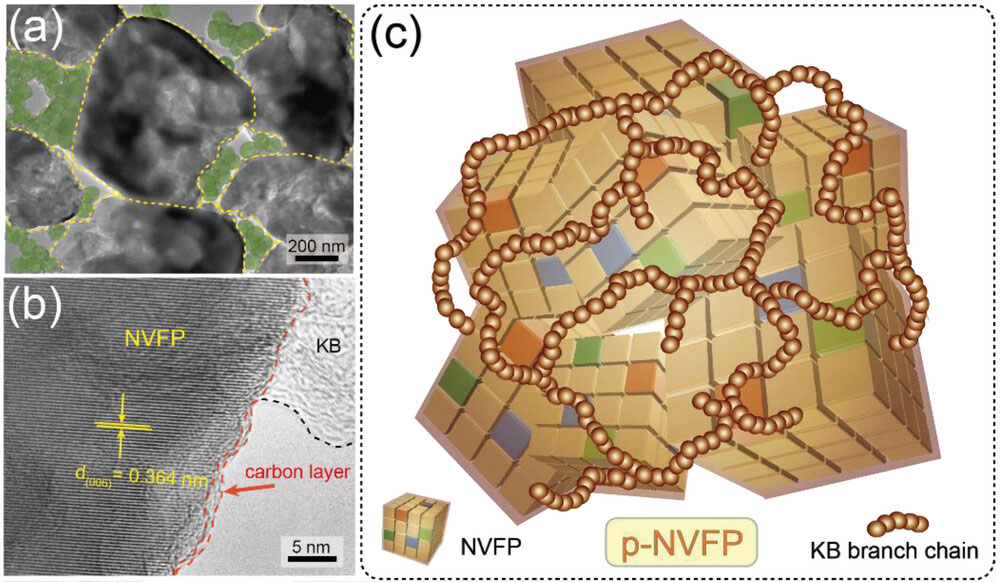
| The researchers synthesized NVFP with a sol-gel method, using citric acid to limit particle growth and coat the particles with carbon. Crucially, they mechanically milled the material with spherical carbon nanoparticles called Ketjen Black (KB), whose unique branching structure links the NVFP particles together in a conductive network. "The KB branch chains encircle the NVFP like pearls on a necklace," Ang explains to Nanowerk, "creating additional electron transport pathways that dramatically increase capacity and durability." | |
| Microscopy and spectroscopy confirmed this "pearl" nanostructure, with chains of KB nanoparticles adhering to the NVFP and boosting its electronic conductivity by nearly an order of magnitude. X-ray diffraction analysis revealed NVFP's robust crystal structure, with the iron and vanadium atoms randomly distributed in octahedral sites. | |
 |
|
| a,b) TEM and HRTEM images of p-NVFP (pearl-like KB branch chains encircling the NVFP). c) Schematic diagram of p-NVFP. (Image: reproduced from DOI:10.1002/advs.202301308, CC BY) | |
| Electrochemical tests revealed the material's exceptional sodium storage capabilities, achieving an impressive 106.8 mAh/g capacity at a moderate rate and retained over 75% of that capacity at a very high 15C rate, far outperforming unmodified NVFP. The structured cathode also demonstrated remarkable stability, retaining 87.7% capacity after 5000 cycles at a 5C rate. | |
| "By buffering the impact of high current densities, the pearl nanostructure allows ultra-stable cycling with minimal degradation," says Ang. | |
| In-situ X-ray diffraction during cycling provided insights into the cathode's charge storage mechanism and structural evolution. Unlike typical NASICON materials, NVFP exhibited an unusual decrease in lattice parameter during initial charging, which the authors attribute to partial oxidation of iron facilitated by the surface interactions with the carbon network. This modulation appears to promote sodium extraction and improve cycling stability, with a volume change of just 3% – among the lowest reported for NASICON electrodes. | |
| Measurements of sodium diffusion kinetics during cycling revealed the NVFP's impressively high ionic conductivity, particularly through the voltage plateaus corresponding to the iron and vanadium redox reactions. The interconnected nanostructure and optimized crystal framework synergistically enhance sodium transport throughout charge and discharge. | |
| To demonstrate practical applicability, the team paired the NVFP cathode with a low-cost hard carbon anode in a full sodium ion cell. The full cell delivered a high 102.5 mAh/g initial capacity and retained 83% of that after 500 cycles at 2C, placing it among the top-performing sodium ion batteries reported to date. | |
| Though challenges remain in matching the energy density of lithium-ion cells, this nanostructured NASICON represents a significant step toward realizing high-performance sodium ion batteries. By combining the multi-electron capacity of vanadium with the abundance of iron in a rationally designed nanostructure, the NTU team has achieved a cathode that exhibits both high energy and ultra-stable long-term cycling. | |
| "With further optimization, our strategy of coupling mixed-metal NASICONs with conductive nanocarbon scaffolds could enable sodium ion batteries that compete with lithium cells on performance while using cheaper, more sustainable materials," Ang concludes. "Such a breakthrough would accelerate the deployment of large-scale rechargeable batteries for electric vehicles and renewable energy storage, helping to power the transition to a clean energy future." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
