| May 27, 2024 | |
Living nanomedicine with hybrid bacteria and nanoparticles enhances tumor therapy |
|
| (Nanowerk Spotlight) Cancer remains one of the most devastating and difficult to treat diseases, responsible for millions of deaths each year worldwide. While early screening and new targeted therapies have improved survival rates for some cancer types in recent decades, many tumors remain highly resistant to current treatments. This is often due to their ability to thrive in hypoxic environments with low oxygen deep within tumor tissues that many drugs cannot effectively reach. | |
| Researchers have long sought to harness bacteria as a Trojan horse to deliver therapeutic payloads deep into tumors. Certain species of bacteria preferentially grow in the hypoxic cores of solid tumors, enabling much deeper penetration than possible with standard nanomedicine drug delivery approaches that rely on passive accumulation. Additionally, some bacteria naturally produce substances toxic to cancer cells. However, maintaining control over bacterial replication and toxicity while achieving a meaningful anti-tumor effect has proven challenging. | |
| Now, scientists from Shanghai University in China report (Advanced Functional Materials, "Engineering Photothermal and H2S-Producing Living Nanomedicine by Bacteria-Enabled Self-Mineralization") an innovative strategy to engineer a hybrid bacterial-nanoparticle system dubbed "Sa@FeS" to launch a multi-pronged attack against tumors from within. | |
 |
|
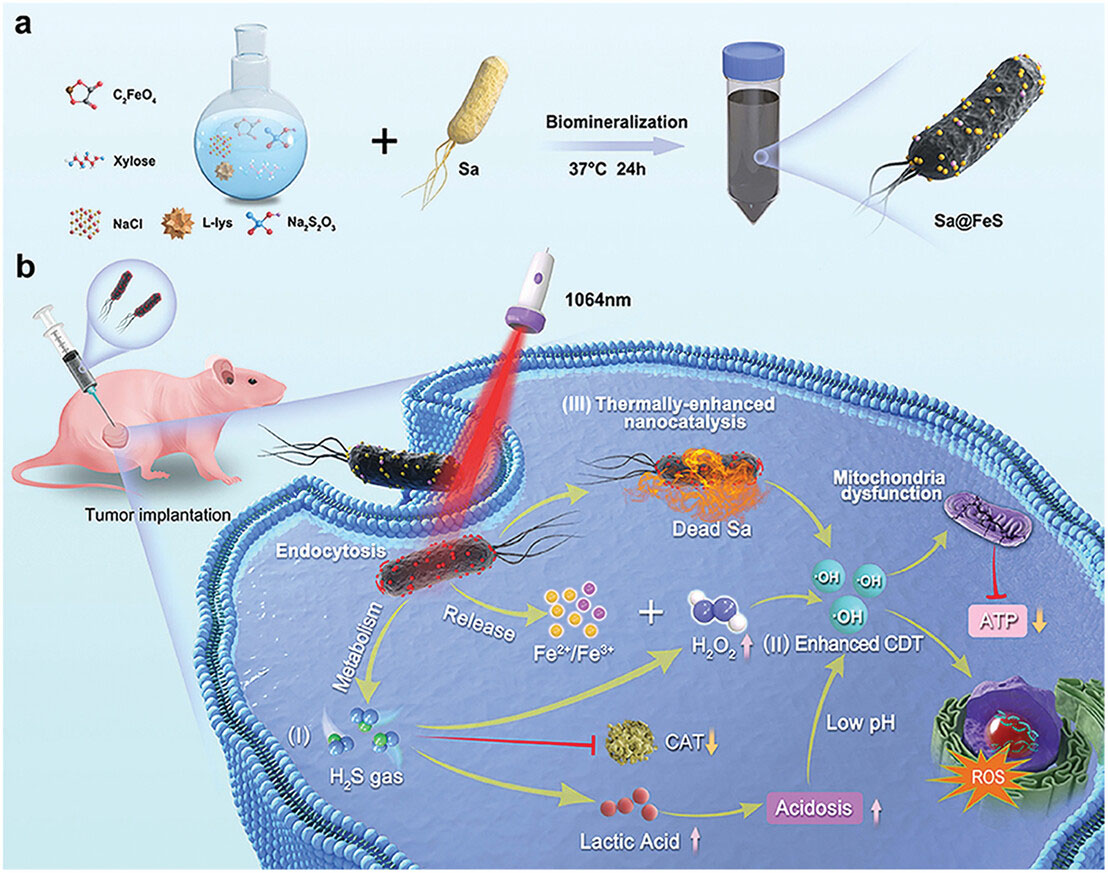
| Systematic illustration of living nanomedicine Sa@FeS for all-in-one antitumor treatment. a) Preparation procedure of Sa@FeS. Exogenous thiosulfate (S2O32−) and ferrous iron were introduced to bacteria liquid medium that sterilized at high temperature with induction cooker. Then the bacteria colony was mixed into the medium, and anaerobically cultured for 24 h to obtain living nanomedicine Sa@FeS. b) Sa@FeS with active targeting for all-in-one tumor therapy. When intratumorally administered, I) H2S gas produced by bacteria could promote CDT via simultaneously suppressing the CAT activity to enhance the H2O2 level and accumulating the lactic acid to induce intracellular acidosis to down-regulate pH of TME. II) Released Fe2+/Fe3+-mediated Fenton reaction could produce cytotoxic ROS against tumor cells. III) FeS NPs on the surface of bacteria could facilitate photothermal therapy (PTT) upon 1064 nm laser irradiation and thermally enhanced CDT. The therapeutic pathways aforementioned above combined efforts to cause the mitochondrial respiratory dysfunction of tumor cancer cells, subsequently blocking the supply of ATP, and inducing the cellular apoptosis or necrosis eventually to ward off cancer. (Reprinted with permission by Wiley-VCH Verlag) (click on image to enlarge) | |
| They start with an attenuated strain of Salmonella typhimurium bacteria, which is drawn to the hypoxic regions in tumors. By feeding the Salmonella specific nutrients, they coax it to biomineralize its cell surface with photothermal iron sulfide nanoparticles without impairing bacterial viability and mobility. | |
| The resulting nanomedicine platform enables three distinct but synergistic therapeutic mechanisms. First, the Salmonella bacteria naturally produce hydrogen sulfide gas, which recent studies show can be directly toxic to cancer cells by damaging DNA, disrupting mitochondrial function, and inhibiting cellular metabolism. Second, upon exposure to near-infrared laser light, the iron sulfide nanoparticles efficiently convert the light to heat, subjecting tumor cells to photothermal ablation. | |
| Most powerfully, the released hydrogen sulfide gas, mildly acidic tumor microenvironment, and photothermal heating work in concert to dramatically amplify the effectiveness of chemodynamic therapy. In this therapy, iron-based nanoparticles convert hydrogen peroxide into highly toxic hydroxyl radicals. | |
| While promising, chemodynamic therapy is often limited by insufficient hydrogen peroxide in tumors. The Sa@FeS therapy overcomes this by using the released hydrogen sulfide to suppress tumor cells' enzymes that break down hydrogen peroxide, causing its levels to build up. Simultaneously, the heating and acidosis accelerate the iron-catalyzed conversion of hydrogen peroxide to hydroxyl radicals. | |
| When injected into mouse models of aggressive triple-negative breast cancer, the Sa@FeS bacteria efficiently penetrated into the hypoxic cores of the tumors and began producing hydrogen sulfide. Subsequent near-infrared laser irradiation then triggered potent photothermal heating to over 70 °C in the tumors. | |
| The multi-pronged Sa@FeS treatment dramatically inhibited tumor growth and induced widespread cancer cell death as confirmed by histological analysis, while laser irradiation alone or bacteria without iron sulfide had little effect. Importantly, the Sa@FeS therapy caused no evident side effects or organ damage in the mice. | |
| The researchers also showed that the photothermal heating could inhibit the viability of the injected bacteria after they accomplished their mission, potentially reducing risks of infection. However, more studies will be needed to further assess safety before clinical translation. | |
| While still at the proof-of-concept stage, this study demonstrates the potential for engineering "smart" living nanomedicines to significantly enhance cancer treatment. By leveraging the innate properties of bacteria and cleverly integrating them with synthetic nanomaterials responsive to external triggers, increasingly sophisticated and targeted therapies with fewer side effects may be possible. | |
| Though highly promising, some key challenges remain before this specific Sa@FeS platform could reach clinical testing. More quantitative studies are needed to characterize nanoparticle loading on the bacteria surfaces and their stability during transport to the tumor. | |
| Additional genetic engineering of the bacteria may also help maximize hydrogen sulfide production and further restrict replication outside the tumor. Long-term safety and immunogenicity also require further scrutiny. | |
| Moreover, the researchers suggest that beyond bacteria, other diverse microorganisms such as fungi and viruses could potentially be engineered for similar therapeutic applications, opening up an even broader horizon for 'living medicines'. Nevertheless, this impressive study lights the way for a new generation of bio-inspired therapies that merge the tools of synthetic biology and nanotechnology to open new fronts in the war against cancer. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
