| May 03, 2024 | |
Advancing responsive colloidal nanomaterials with virus-polymer crystals |
|
| (Nanowerk Spotlight) Researchers have demonstrated a simple yet powerful method for creating highly organized nanomaterials by combining bacteriophages – viruses that infect bacteria – with synthetic polymers. The resulting colloidal crystals, which are ordered structures composed of particles dispersed in a medium, can be tuned by adjusting pH levels and polymer chain length. This allows the properties of the material to be customized for specific applications. | |
| Notably, this assembly process occurs spontaneously without chemically modifying the viruses, setting it apart from previous techniques that require laborious genetic alterations or additional processing steps. | |
| Viruses have long captivated scientists' imaginations as potential building blocks for advanced materials, thanks to their well-defined shapes, sizes and surface properties. Bacteriophages in particular are attractive candidates because they can be bioengineered and produced at large scales. However, organizing viral particles into complex hierarchical structures with tunable properties has remained an elusive goal. | |
| In a new study, reported in Advanced Functional Materials ("pH-Responsive Virus-Based Colloidal Crystals for Advanced Material Platforms"), researchers from the University of Fribourg and collaborating institutions across Europe report the formation of colloidal crystals through the spontaneous assembly of the Qbeta bacteriophage with a synthetic polycation called pMETAC. Qbeta is a small icosahedral virus about 29 nanometers in diameter that infects E. coli bacteria. Its negative surface charge and homogeneous size distribution make it an ideal model system. | |
 |
|
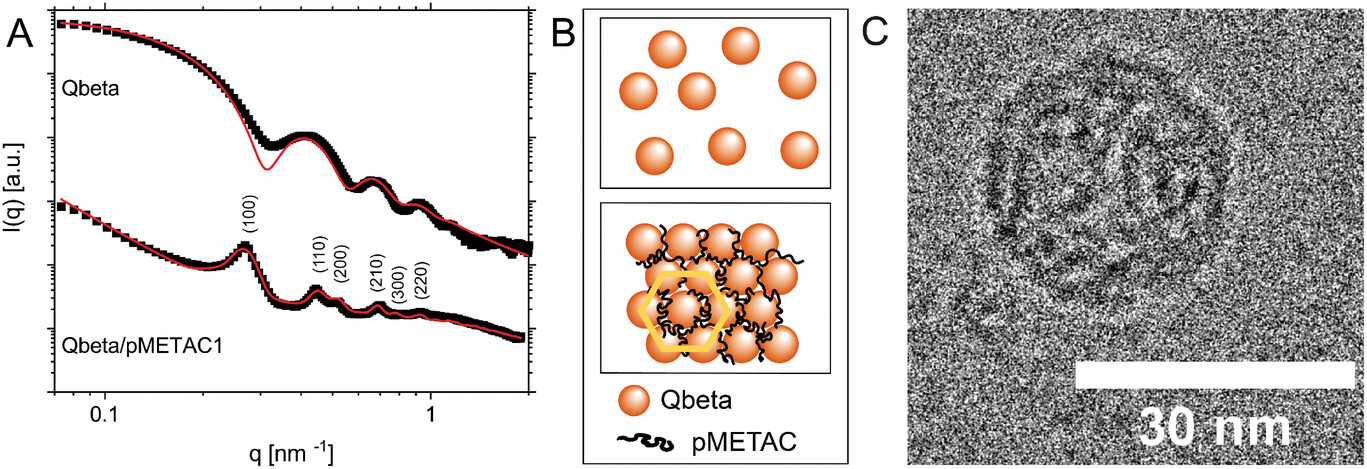
| Qbeta/pMETAC1 (1:50 weight ratio) self-assembly at pH 7.0. The curves were displaced vertically for better visibility. A) Black squares represent the experimental SAXS data; red lines are the best possible fits of Equation S9 (Supporting Information) to the experimental SAXS data of Qbeta and the Qbeta/pMETAC1 assembly. The peaks in the SAXS curve are further identified by their Miller indices (hkl). B) is an artistic representation of the data showing the Qbeta in a hexagonal arrangement, marked in yellow, with polymers on the surface and in the voids. It is worth noting that the icosahedral Qbeta is represented as spheres. C) Cryo-TEM image showing icosahedral Qbeta with a diameter of ≈29 nm at pH 7.0 in VDB. (Image: Reprinted from DOI:10.1002/adfm.202402257, CC BY) | |
| When mixed together, Qbeta and pMETAC rapidly self-assemble into micron-sized aggregates with highly ordered internal structures, as revealed by small angle X-ray scattering (SAXS) and atomic force microscopy. Strikingly, the aggregates exhibit hexagonal packing of the viral particles – a rare geometry for spherical colloids that typically prefer cubic arrangements. The researchers attribute this unique hexagonal lattice to patchy electrostatic interactions between the polycation and the virus surface. | |
| By systematically varying the pH and pMETAC chain length, the team mapped out the conditions for forming the colloidal crystals. At neutral to alkaline pH, Qbeta has a strong negative charge that drives adsorption of the positively charged pMETAC. However, lowering the pH below 7 reduces the charge difference, causing the aggregates to disassemble. Using a longer pMETAC chain also leads to more disordered structures, likely due to bridging interactions between viruses. | |
| Importantly, the Qbeta particles maintain their structural integrity and infectious activity within the colloidal crystals. The supramolecular assemblies can be easily separated from solution by centrifugation, allowing the active viruses to be concentrated and purified for further use. The researchers also show that the process is fully reversible – adding salt or lowering pH triggers the aggregates to dissociate and release the individual viral building blocks. | |
| Besides illuminating the fundamental forces governing nanoparticle self-assembly, the study presents a practical method for organizing functional biomolecules into responsive materials with emergent properties. The resulting virus-polymer composites are stable up to 60 –C and can potentially encapsulate drugs or enzymes within the viral capsid for controlled release. | |
| One exciting application is to use bacteriophages as natural antimicrobials to combat the growing threat of antibiotic resistance. Concentrating phages in a structured colloidal gel could increase their efficacy and provide sustained delivery to infection sites. The environmentally triggered disassembly could further allow the phages to penetrate bacterial biofilms and access hidden reservoirs of pathogens. | |
| As a nanoscale engineering platform, the ability to assemble viruses in crystalline lattices also opens possibilities for developing new biosensors, catalysts and optical materials. The well-defined placement of functional proteins on the viral surface could enable the precise spatial organization of enzymes for multi-step reactions, similar to natural signaling cascades. Incorporating fluorescent or plasmonic nanoparticles could allow the creation of responsive photonic crystals. | |
| While this study represents a major advance, the authors note there are still challenges to overcome before these materials are ready for commercial use. Scaling up the assembly process while maintaining quality control will require further optimization. The environmental sensitivity of the ionic interactions may also limit the range of conditions in which the crystals are stable. | |
| The fact that this assembly process occurs spontaneously in aqueous conditions, without the need for genetic or chemical modification of the viruses, makes it particularly attractive for large-scale manufacturing. The authors envision their approach being extended to other viruses and biopolymers, greatly expanding the available toolkit for fabricating advanced biomaterials. | |
| With further development, virus-based colloidal crystals may become the key to unlocking nature's patterning secrets for human benefit. By bridging biology and materials science, such hybrid living-nonliving systems could transform fields ranging from medicine to energy conversion and environmental remediation. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
