| Aug 08, 2024 |
Precise stirring conditions key to optimizing nanostructure synthesis
(Nanowerk News) Stirring allows for homogenization and efficient gas exchange – this fact has been known for decades. Controlling the stirring rate during the nanocluster synthesis is pivotal in achieving nanostructures with well-defined sizes, structures, optical properties, and stability. This insight was highlighted in a recent study (Small, "Stirring-Controlled Synthesis of Ultrastable, Fluorescent Silver Nanoclusters") led by Director Bartosz A. Grzybowski of the Center for Algorithmic and Robotized Synthesis within the Institute for Basic Science.
|
|
As the case study, the group chose the synthesis of fluorescent silver nanoclusters in the classical sodium borohydride/glutathione system. The researchers realized that the delivery of oxygen to the synthetic mixture at a proper time and rate can be a make-or-break factor for the result of the synthesis.
|
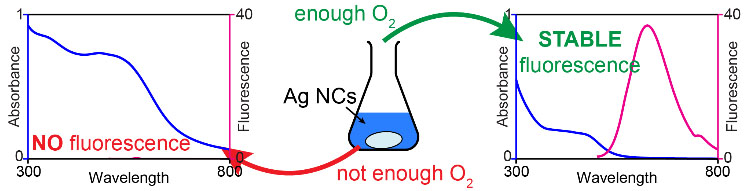 |
| If the mixture of silver nitrate, glutathione, and sodium borohydride is stirred under an atmosphere not containing enough oxygen, the product is a blend of polydisperse and not fluorescent particles (left). Only when there is enough oxygen delivered, the reaction mixture yields stable and strongly fluorescent silver nanoclusters. (Image: IBS)
|
|
It was possible to precisely tune the properties of obtained structures – from unstable and non-fluorescent to highly fluorescent– just by controlling the stirring conditions. Even a very small change in the stirring bar size, from 25 by 12 mm to 20 by 10 mm, while keeping the same stirring rate, systematically resulted in a non-fluorescent product.
|
|
Moreover, oxygen was determined as a factor dictating the structure and functional properties of silver nanoclusters. Delivering copious amounts of oxygen using precise stirring conditions resulted in the creation of fluorescent silver nanoclusters that boast remarkable stability, which retained fluorescence for over two years.
|
|
“As a chemist, I was quite puzzled that oxygen did not simply "ruin"/oxidize silver – instead, the fluorescence became so lasting because oxygen incorporated into the silver lattice of the forming nanoclusters,” said Director Grzybowski, the head of the group.
|
|
The unprecedented stability of these silver nanoclusters may open the door for wider use in catalysis, biosensing, or bioimaging.
|
|
This study enhances the understanding of the relationship between the structure and properties of nanostructures, paving the way for a more flexible design of nanomaterials with tailored properties and broadening the scope of their potential uses.
|

