| Jul 24, 2024 |
Nanoparticle-coated catalyst boosts sustainable acetate production
(Nanowerk News) Acetic acid, also known as acetate, and other products that can be developed from acetic acid are used in a variety of industries, from food production to medicine to agriculture. Currently, acetate production uses a significant amount of energy and results in harmful waste products. The efficient and sustainable production of acetate is an important target for researchers interested in improving industrial sustainability.
|
|
A paper published in Carbon Future ("CO2 electroreduction to acetate by enhanced tandem effects of surface intermediate over Co3O4 supported polyaniline catalyst") outlines a method using a polyaniline catalyst with cobalt oxide nanoparticles to produce acetate through carbon dioxide electroreduction.
|
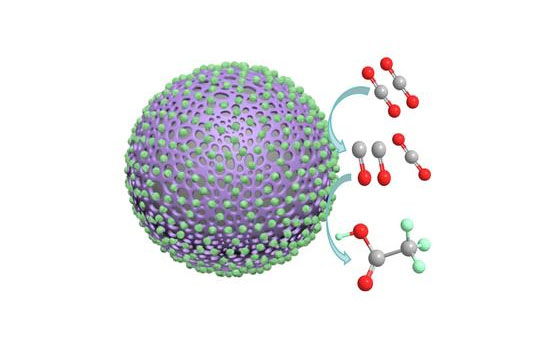 |
| This image shows a polyaniline catalyst coated in cobalt oxide nanoparticles and demonstrates how the catalyst facilitates the conversion of carbon dioxide to carbon monoxide to acetate. (Image: Carbon Future)
|
|
“The polyaniline catalyst with cobalt oxide nanoparticles has two components—polyaniline as a continuous material and cobalt oxide as nanoparticles dispersed on the polyaniline. This cooperative structure makes a highly selective catalyst that can produce acetate during carbon dioxide electroreduction. Cobalt oxide is in charge to produce carbon monoxide intermediate and then pass them to polyaniline, where acetate is formed by electroreduction,” said Liwen Wang, a professor at the School of Chemistry and Chemical Engineering at Nanjing University in Nanjing, China.
|
|
Polyaniline is a conducting polymer that has been proven to be a highly selective catalyst used in the generation of other carbon products. This study looks at the role of polyaniline and the mechanism of carbon dioxide electroreduction over the polyaniline surface. A higher carbon monoxide concentration on the polyaniline enhances carbon to carbon coupling on the catalyst surface. Adding cobalt oxide nanoparticles as a supplementary catalyst creates a tandem reaction that is highly selective for acetate.
|
|
“This configuration facilitates a higher local carbon monoxide concentration over polyaniline and enhances carbon-to-carbon coupling. The non-metallic polyaniline material can provide excellent performance in electrocatalysts,” said Wang. She went on to describe the synergy between the polyaniline material and the cobalt oxide nanoparticles. “The polyaniline provides available active sites for increasing the carbon-to-carbon coupling, while the cobalt oxide nanoparticles offer a large number of carbon monoxide intermediates.”
|
|
To measure how well the polyaniline/cobalt oxide catalyst worked, researchers also prepared two control samples: a polyaniline catalyst without cobalt oxide and a cobalt oxide catalyst. The polyaniline/cobalt oxide catalyst had improved crystallization with a larger crystal size and uniform deposits of the cobalt oxide nanoparticles. The polyaniline coating also meant there was a higher surface area, which most likely meant there were more sites for the electro-conversion of carbon dioxide. A test called an electron pragmatic resonance (EPR) measurement showed that the polyaniline/cobalt oxide catalyst had more oxygen vacancies, which trap the carbon dioxide and allow the proton-electron transfers necessary for the transformation.
|
|
Additional testing went on to confirm that it was not the cobalt oxide or the polyaniline alone that accounted for the improved performance of the polyaniline/cobalt oxide catalyst. It was the synergistic nature of the polyaniline and cobalt oxide together.
|
|
Looking ahead, researchers hope to continue improving the synergistic performance between polyaniline and cobalt oxide on this catalyst. “The next step is to optimize the catalyst system, enhancing the tandem effect for better performance. The ultimate goal is the direct electrosynthesis of acetate using carbon dioxide and water as raw materials,” said Wang.
|

