| May 29, 2024 |
Solving the problems of proton-conducting perovskites for next-generation fuel cells
(Nanowerk News) In line with global efforts towards cleaner energy technologies, fuel cells may soon become an indispensable tool for converting chemical energy – stored in the form of hydrogen or other fuels – into electrical energy. Among the various types of fuel cells being actively researched, those that use solid electrolytes rather than liquid ones have inherent safety and stability advantages.
|
|
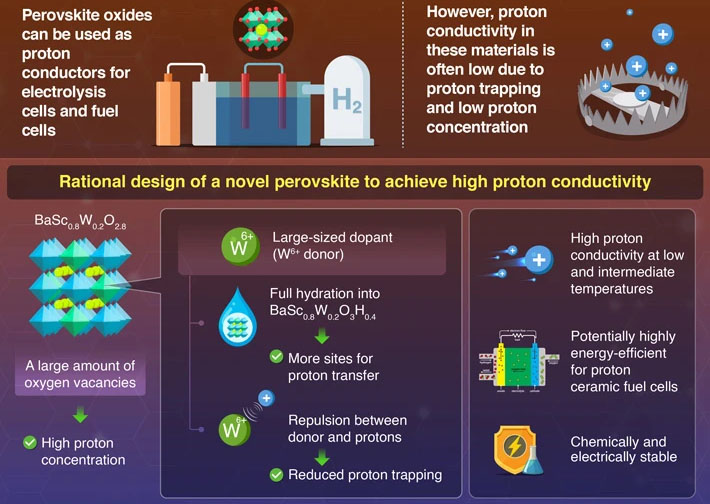
In particular, protonic ceramic fuel cells (PCFCs) have attracted special attention among scientists. These devices do not operate via the conduction of oxide ions (O2−) but light protons (H+) with smaller valence. A key feature of PCFCs is their ability to function at low and intermediate temperatures in the range of 50–500 °C. However, PCFCs based on perovskite electrolytes reported thus far suffer from low proton conductivity at low and intermediate temperatures.
|
|
In a recent study (Journal of Materials Chemistry A, "High proton conduction by full hydration in highly oxygen deficient perovskite"), a research team led by Professor Masamoto Yashima from Tokyo Institute of Technology (Tokyo Tech), in collaboration with High Energy Accelerator Research Organization (KEK), has set out to address this limitation of perovskite-based proton conductors.
|
 |
| Innovations in perovskite design for proton conductors. (Image: Tokyo Tech)
|
|
But why is the conductivity of the conventional perovskite-type proton conductors so low? "A major problem with the conventional proton conductors is a phenomenon known as proton trapping, in which protons are trapped by acceptor dopant via electrostatic attraction between the dopant and proton," explains Yashima. "Another major problem among such proton conductors would also be their low proton concentration due to the small amount of oxygen vacancies."
|
|
To tackle these issues, the researchers developed a highly oxygen-deficient perovskite, namely BaScO2.5 doped with W6+ cations, or BaSc0.8W0.2O2.8. Thanks to its large amounts of oxygen vacancies, this material has a higher proton concentration than other proton-conducting perovskites. However, since proton hopping occurs between oxygen atoms, the oxygen vacancies would lower proton conductivity rather than increase it.
|
|
This problem was solved by full hydration of the perovskite, turning it into BaSc0.8W0.2O3H0.4. Because of the large size of the W6+ dopant, the perovskite has a larger lattice volume, which means it can take up more water molecules than those doped with other cations such as small Mo6+. The high water uptake facilitates high proton conductivity by further increasing the proton concentration.
|
|
As for proton trapping, the high positive charge of the W6+ dopant leads to a stronger repulsion with protons, which are also positively charged. This effect was confirmed through ab initio molecular dynamics simulations, which revealed the migration pathways of protons near the Sc cation when transporting across the material. The repulsion indicates reduced proton trapping by the W6+ dopant, which leads to the high proton conductivity at low and intermediate temperatures.
|
|
Taken together, the insights provided by this study could help establish fundamental design principles for proton-conducting perovskites. "The stabilization of perovskites with disordered intrinsic oxygen vacancies and full hydration enabled by doping of large donor dopant could be an effective strategy towards next-generation proton conductors," remarks Yashima.
|
|
In addition to PCFCs, proton conductors are also needed in proton-conducting electrolysis cells (PCECs), which can efficiently utilize electricity. Both of these technologies will be essential in the near future as we collectively strive towards sustainability through novel proton conductors.
|

