Zeta Potential Measurements: Assessing Nanoparticle Stability and Behavior
What is Zeta Potential?
Zeta potential is a key indicator of the stability of colloidal dispersions and nanoparticle suspensions. It measures the electrical potential difference between the bulk of the liquid and the stationary layer of fluid attached to the dispersed particle. This potential is influenced by the surface charge of the particle and the composition of the surrounding medium, including its pH, ionic strength, and the presence of adsorbed species.
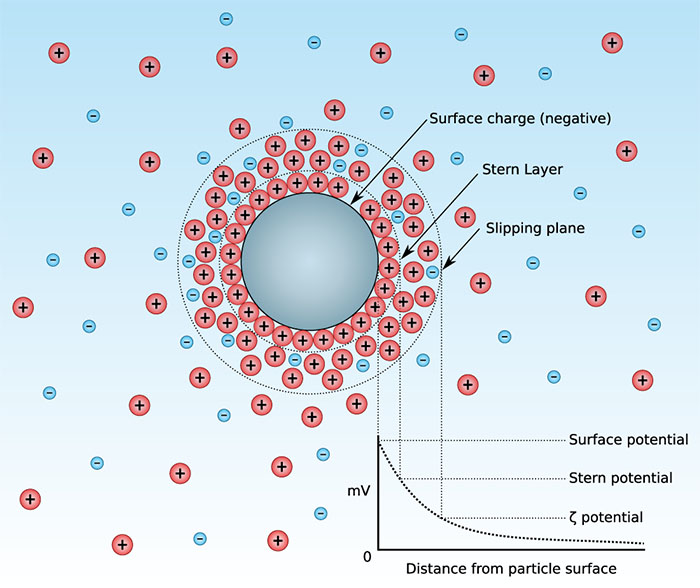
The Electrical Double Layer
When a particle is dispersed in a liquid, it typically acquires a surface charge due to ionization, ion adsorption, or ion dissolution. This surface charge attracts counter-ions from the surrounding medium, forming an electrical double layer around the particle. The double layer consists of two regions:
- Stern Layer: The inner region where counter-ions are strongly bound to the particle surface.
- Diffuse Layer: The outer region where ions are less firmly associated and their distribution is determined by a balance of electrostatic forces and random thermal motion.
Measuring Zeta Potential
Zeta potential is typically measured using electrophoretic light scattering techniques, such as laser Doppler velocimetry or phase analysis light scattering. In these methods, an electric field is applied to the sample, causing the charged particles to move toward the electrode of opposite charge. The velocity of this motion, known as the electrophoretic mobility, is measured and used to calculate the zeta potential using the Henry equation:
Where:
- μ is the electrophoretic mobility
- ε is the dielectric constant of the medium
- ζ is the zeta potential
- f(κa) is Henry's function, which depends on the particle size and the thickness of the electrical double layer
- η is the viscosity of the medium
Interpreting Zeta Potential Values
The magnitude of the zeta potential provides an indication of the stability of the colloidal system:
- High Zeta Potential (> +30 mV or < -30 mV): Particles with high absolute zeta potential values are considered stable, as the strong electrostatic repulsion between particles prevents aggregation.
- Low Zeta Potential (-30 mV < ζ < +30 mV): Particles with low absolute zeta potential values are prone to aggregation and flocculation, as the electrostatic repulsion is not sufficient to overcome the attractive van der Waals forces.
The sign of the zeta potential (positive or negative) indicates the surface charge of the particles and can provide insights into their behavior and interactions with other components in the system.
Factors Affecting Zeta Potential
Several factors can influence the zeta potential of nanoparticles:
- pH: Changes in pH can affect the surface charge of particles by altering the protonation state of surface groups. The isoelectric point (IEP) is the pH at which the zeta potential is zero.
- Ionic Strength: Increasing the ionic strength of the medium can compress the electrical double layer, reducing the absolute value of the zeta potential.
- Adsorbed Species: The adsorption of ions, surfactants, or polymers onto the particle surface can modify the surface charge and zeta potential.
- Particle Size and Shape: The size and shape of the particles can affect the thickness of the electrical double layer and, consequently, the zeta potential.
Applications of Zeta Potential Measurements
Zeta potential measurements have numerous applications in nanotechnology and related fields:
- Stability Assessment: Zeta potential is used to evaluate the stability of colloidal suspensions, nanoparticle dispersions, and emulsions. It helps predict the shelf life and performance of these systems.
- Formulation Optimization: By monitoring the zeta potential, researchers can optimize the composition of nanoparticle formulations to achieve desired stability, dispersibility, and functionality.
- Surface Modification: Zeta potential measurements can be used to assess the effectiveness of surface modification techniques, such as functionalization or coating, in altering the surface properties of nanoparticles.
- Interaction Studies: Zeta potential can provide insights into the interactions between nanoparticles and other components in a system, such as proteins, cells, or surfaces, which is crucial for applications in drug delivery, biosensing, and environmental remediation.
Challenges and Limitations
While zeta potential measurements are widely used and provide valuable information, there are some challenges and limitations to consider:
- Sample Preparation: Zeta potential measurements are sensitive to sample preparation, including the choice of dispersant, pH, and ionic strength. Careful control of these parameters is necessary for reliable and reproducible results.
- Concentration Effects: The accuracy of zeta potential measurements can be affected by particle concentration, as high concentrations can lead to multiple scattering effects and electrode polarization.
- Non-spherical Particles: The interpretation of zeta potential for non-spherical particles, such as rods or platelets, can be more complex due to their anisotropic shape and surface properties.
- Heterogeneous Samples: Zeta potential measurements provide an average value for the entire sample, which may not capture the heterogeneity in surface charge or particle size within the sample.
Despite these challenges, zeta potential remains a powerful tool for characterizing nanoparticle surfaces and predicting their behavior in various applications.
Further Reading
Catalysis Today, Zeta potential as a tool for functional materials development
Environmental Science: Nano, Guidance to improve the scientific value of zeta-potential measurements in nanoEHS
