Tissue Engineering Scaffolds: 3D Printed Biomaterials Enabling for Regenerative Medicine
What are Tissue Engineering Scaffolds?
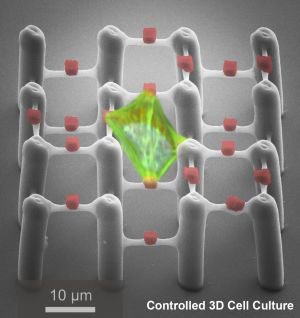
Tissue engineering scaffolds are three-dimensional structures designed to support cell growth, migration, and differentiation for the regeneration of damaged or diseased tissues. These scaffolds act as temporary extracellular matrices, providing a suitable environment for cells to attach, proliferate, and form new tissue. Scaffolds can be made from various biocompatible materials and can be customized to mimic the structure and properties of the native tissue being regenerated.
Key Properties of Tissue Engineering Scaffolds
Effective tissue engineering scaffolds should possess several key properties to support tissue regeneration:
- Biocompatibility: Scaffolds must be made from materials that are non-toxic, non-immunogenic, and compatible with the body to avoid adverse reactions and promote cell survival.
- Biodegradability: Scaffolds should degrade at a controlled rate that matches the tissue regeneration process, allowing the newly formed tissue to replace the scaffold over time.
- Porosity: Scaffolds must have an interconnected porous structure to facilitate cell infiltration, nutrient transport, and waste removal. Pore size and distribution can be tailored to suit specific tissue requirements.
- Mechanical Properties: Scaffolds should have mechanical properties similar to the native tissue to provide structural support and withstand physiological stresses during the regeneration process.
- Surface Chemistry: The surface chemistry of scaffolds can be modified to enhance cell adhesion, proliferation, and differentiation by incorporating bioactive molecules or functional groups.
Materials Used in Tissue Engineering Scaffolds
Various materials can be used to fabricate tissue engineering scaffolds, depending on the target tissue and desired properties. Common materials include:
Natural Polymers
Natural polymers, such as collagen, gelatin, hyaluronic acid, and chitosan, are derived from biological sources and offer excellent biocompatibility and biodegradability. These materials can closely mimic the native extracellular matrix and promote cell adhesion and growth. However, they may have limited mechanical strength and may be difficult to process into complex shapes.
Synthetic Polymers
Synthetic polymers, such as poly(lactic acid) (PLA), poly(glycolic acid) (PGA), and their copolymers, offer tunable mechanical properties, degradation rates, and ease of processing. These materials can be readily fabricated into various scaffold geometries using techniques like electrospinning, 3D printing, and solvent casting. However, they may lack the intrinsic bioactivity of natural polymers and may require surface modifications to improve cell interactions.
Ceramics and Bioactive Glasses
Ceramics, such as hydroxyapatite and tricalcium phosphate, and bioactive glasses are commonly used for bone tissue engineering. These materials have excellent biocompatibility, osteoconductivity, and can stimulate bone formation. However, they are brittle and may have limited processability compared to polymers.
Composite Scaffolds
Composite scaffolds combine two or more materials to leverage their individual strengths and overcome their limitations. For example, combining a natural polymer with a synthetic polymer can provide a scaffold with both bioactivity and mechanical strength. Nanocomposites, which incorporate nanomaterials like nanofibers or nanoparticles, can further enhance the biological and mechanical properties of scaffolds.
Fabrication Techniques for Tissue Engineering Scaffolds
Several fabrication techniques can be used to create tissue engineering scaffolds with desired architectures and properties:
Electrospinning
Electrospinning is a versatile technique that uses an electric field to produce ultrafine fibers from polymer solutions or melts. This method can create highly porous scaffolds with interconnected pores and high surface-to-volume ratios. Electrospun scaffolds closely mimic the fibrous structure of the extracellular matrix and can be easily functionalized with bioactive molecules.
3D Printing
3D printing, also known as additive manufacturing, enables the fabrication of scaffolds with precise control over geometry, pore size, and spatial distribution of materials. Various 3D printing techniques, such as fused deposition modeling (FDM), stereolithography (SLA), and selective laser sintering (SLS), can be used to create patient-specific scaffolds based on medical imaging data.
Freeze-drying
Freeze-drying, or lyophilization, is a technique that involves freezing a polymer solution followed by sublimation of the solvent under vacuum. This process creates highly porous scaffolds with interconnected pores. Freeze-drying is particularly suitable for natural polymers and can maintain their biological activity.
Gas Foaming
Gas foaming uses high-pressure gas, typically carbon dioxide, to create porous scaffolds. The gas is dissolved into a polymer solution or melt, and upon release of the pressure, the gas expands and forms pores within the polymer matrix. This technique can produce scaffolds with high porosity and without the use of organic solvents.
Applications of Tissue Engineering Scaffolds
Tissue engineering scaffolds have a wide range of applications in regenerative medicine, including:
- Bone Regeneration: Scaffolds can be used to regenerate bone defects caused by injury, disease, or congenital disorders. Scaffolds made from ceramics, bioactive glasses, or polymers can provide mechanical support and stimulate bone formation.
- Cartilage Repair: Scaffolds can be used to regenerate articular cartilage damaged by osteoarthritis or injury. Hydrogel scaffolds made from natural polymers like hyaluronic acid or collagen can provide a suitable environment for chondrocyte growth and cartilage matrix production.
- Skin Regeneration: Scaffolds can be used to treat deep wounds, burns, or chronic ulcers. Collagen-based scaffolds or decellularized extracellular matrix scaffolds can promote skin cell growth and wound healing.
- Vascular Grafts: Scaffolds can be used to create small-diameter vascular grafts for the treatment of cardiovascular diseases. Electrospun scaffolds made from biodegradable polymers can mimic the structure and mechanical properties of native blood vessels.
- Neural Tissue Engineering: Scaffolds can be used to regenerate damaged nerve tissue or spinal cord injuries. Aligned nanofiber scaffolds or hydrogel scaffolds incorporating neurotrophic factors can guide axonal growth and promote neural regeneration.
Challenges and Future Perspectives
Despite the significant progress in tissue engineering scaffold design and fabrication, several challenges remain. One major challenge is the vascularization of scaffolds, particularly for large tissue constructs. Developing strategies to promote rapid vascularization and integration with the host tissue is crucial for the success of tissue engineering approaches.
Another challenge is the long-term stability and functionality of the regenerated tissues. Scaffolds should not only support initial tissue formation but also facilitate the maturation and remodeling of the tissue over time. Incorporating bioactive molecules, growth factors, or stem cells into scaffolds can help guide tissue development and improve long-term outcomes.
Future research in tissue engineering scaffolds will focus on developing advanced materials with enhanced biological properties, such as stimuli-responsive polymers or self-healing hydrogels. The integration of nanotechnology, such as the use of nanofibers, nanoparticles, or nanopatterning, can further improve the biomimetic properties of scaffolds and control cell behavior at the nanoscale.
Moreover, the combination of tissue engineering scaffolds with other technologies, such as bioreactors, microfluidics, or 3D bioprinting, can enable the creation of more complex and functional tissue constructs. The development of personalized scaffolds based on patient-specific data and the use of artificial intelligence and machine learning for scaffold design optimization are also promising areas for future research.
Further Reading
Macromolecules, Electrospun Scaffolds for Tissue Engineering: A Review
Materials Science and Engineering: C, Nanoengineered Scaffolds for Tissue Engineering: A Review
Nanoengineered Biomaterials for Regenerative Medicine, Nanoengineered biomaterials for vascular tissue engineering
