Electronegativity: Key Property of Periodic Table Elements and Its Impact on Chemical Bonding
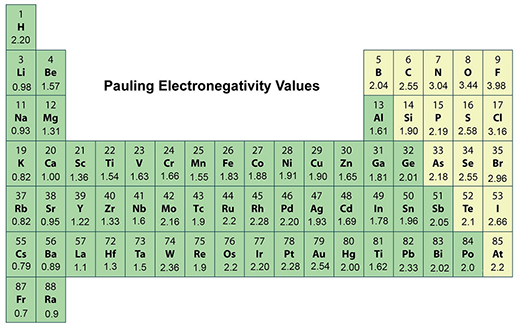
Electronegativity, an essential characteristic of all elements in the periodic table, measures an atom's ability to attract electrons towards itself within a chemical bond. This vital theoretical concept in chemistry, first introduced by American chemist Linus Pauling in 1932 as ‘the power of an atom in a molecule to attract electrons to itself’, has played a significant role in shaping the fields of structural chemistry, solid-state chemistry, and physics. Pauling's electronegativity scale, the most widely used and found in every chemistry textbook, ranges from 0.7 for cesium to 4.0 for fluorine.
The higher an element's electronegativity, the stronger its ability to attract electrons and create a partial negative charge. In a chemical bond, the more electronegative atom gains extra electrons, becoming negatively charged, while the less electronegative atom loses electrons and becomes positively charged. The difference in electronegativity between two bonded atoms determines the bond's polarity, with a large difference indicating a polar covalent bond (e.g., H-F bond) and a small difference indicating a non-polar covalent bond (e.g., C-C bond).
Electronegativity is crucial for explaining various aspects, ranging from the energy of chemical bonds to the (in)stability of chemical compounds. It is expected to correlate with numerous physical properties of materials, including mechanical (such as hardness) and electronic properties, as well as optical properties (like color).
Chemists have come up with various definitions and scales of electronegativity. Yet Pauling's scale is the first and the most common one, present in every chemistry textbook.
Definition of electronegativity
Electronegativity is defined as the tendency of an atom to attract electron density, i.e., to polarize the chemical bond. The most widely used scale is the thermo-chemical Pauling’s scale, where electronegativities have units of eV1/2.
Electronegativity is a derived quantity, so it is not directly measurable. It is relative to hydrogen which was assigned a value of 2.20 on the Pauling scale.
Examples of electronegativity
In an oxygen (O2) molecule, both atoms have the same electronegativity. The electrons in the covalent bond are shared equally between the two oxygen atoms.
The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the Cl than to the H in the HCl molecule.
How to determine electronegativity
Pauling deduced his electronegativity values from thermo-chemistry using the energies of some chemical bonds. He proposed the simplest formula to calculate a bond's stabilization due to the difference in electronegativity between the atoms. It later transpired that the predictions made with Pauling's scale had a rather low accuracy.
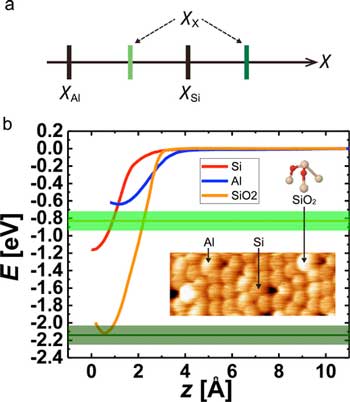
Electronegativity determination of surface atoms solely by experiments (Source). (a) Magnitude relationships of electronegativities for Si,Al, and unknown X atoms. (b) Short-range E(z) curves obtained on Si (red), Al (blue) adatoms, and SiO2 (orange), respectively. The expected values of bond energy on SiO2 acquired by the Al tip are represented by light and dark green lines with their error bands. The inset shows a typical topographic AFM image of Si, Al adatoms, and SiO2 on the Si(111)-(7x7) surface. The ball-and-stick model of SiO2 represents Si in cream and O in red. (Reprinted with permission by American Chemical Society)
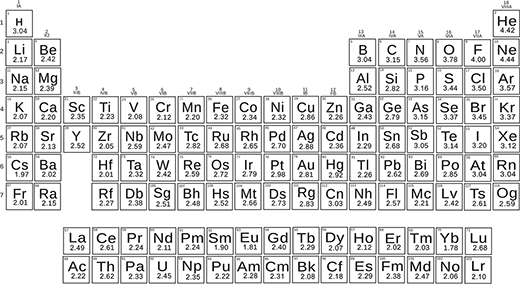
More recently, scientists modified Pauling's formula and redefined the electronegativities of elements. This has led to the creation of a new scale of electronegativity (published in Nature Communications: Thermo-chemical electronegativities of the elements).
"It all started when we decided to calculate Pauling's electronegativities under pressure,” explains Prof Artem R. Organov, corresponding author of the paper. “The chemistry of high pressures is quite exotic. Still, you will likely be able to understand a lot of things once you find out how the electronegativities of elements change under pressure. We used Pauling's definition to calculate electronegativity under normal conditions. We were amazed to discover that his scale did not match either theoretical or experimental bond energies for significantly ionic molecules.”
He continues: “Moreover, many publications in chemical literature mention this inconsistency, but none offer a consistent solution. I realized that the root cause was that Pauling treated the molecule's ionic stabilization as an additive effect. If we consider it a multiplicative effect, many drawbacks will be removed.”
With the new formula and experimental energies of chemical bonds, the scientists determined the electronegativities of all elements. They obtained a beautiful scale that works both for small and large differences in electronegativity.
The new scale uses electronegativity as a dimensionless quantity, which is very practical and accurately reproduces both molecules' energies and chemical reactions.
Periodic table of the new thermo-chemical electronegativ values.
Most and least electronegative elements
The periodic table's most electronegative element is fluorine (3.98 on the Pauling scale and top right in the periodic table). The least electronegative element is cesium (0.79 on the Pauling scale and left in the second row from the bottom in the periodic table).
Note that older texts list both francium and cesium as least electronegative at 0.7, but the value for cesium was experimentally revised to the 0.79 value. There is no experimental data for francium, but its ionization energy is higher than that of cesium, so it is expected that francium is slightly more electronegative.
Electronegativity periodic table trend
Electronegativity generally increases as you move from left to right across a period in the periodic table. It decreases as you move down a group.
This means that the most electronegative elements are found on the top right of the periodic table, while the least electronegative elements are found on the bottom left.
Electronegativity and ionization energy follow the same periodic table trend. Elements that have low ionization energies tend to have low electronegativities. The nuclei of these atoms don't exert a strong pull on electrons. Similarly, elements that have high ionization energies tend to have high electronegativity values. The atomic nucleus exerts a strong pull on electrons.