Transgenesis: Engineering Organisms with Foreign Genes
What is Transgenesis?
Transgenesis is a biotechnological process that involves the introduction of foreign genetic material (transgenes) into a living organism, resulting in the creation of a transgenic organism. This technique allows for the stable integration and expression of genes from one species into the genome of another, enabling the development of organisms with novel traits and characteristics.
History of Transgenesis
The field of transgenesis has a rich history, with several key milestones:
- 1974: Rudolf Jaenisch and Beatrice Mintz create the first transgenic mice by injecting viral DNA into early mouse embryos.
- 1982: Ralph Brinster and Richard Palmiter generate transgenic mice expressing the rat growth hormone gene, leading to the development of "super mice" with enhanced growth.
- 1986: The first transgenic plant, tobacco, is created by introducing a bacterial antibiotic resistance gene using Agrobacterium-mediated transformation.
- 1997: The sheep Polly is cloned from a cell containing a human gene, marking a significant milestone in animal pharming, although earlier examples of animal pharming exist.
- 2010: The AquAdvantage salmon, the first transgenic animal for human consumption, is created with enhanced growth properties.
These milestones have paved the way for the rapid development of transgenesis and its applications in various fields.
Key Steps in Transgenesis
The process of transgenesis involves several crucial steps:
- Identification and Isolation of the Transgene: The first step is to identify the gene of interest (transgene) that confers the desired trait. This gene is then isolated from the donor organism using molecular biology techniques such as PCR or gene synthesis.
- Vector Construction: The isolated transgene is inserted into a suitable vector, which is a DNA molecule that can replicate and express the transgene in the host organism. Common vectors include plasmids, viruses, and artificial chromosomes.
- Delivery of the Transgene: The vector containing the transgene is then delivered into the host organism's cells. Various methods can be used for transgene delivery, such as microinjection, electroporation, or viral transduction, depending on the target species and cell type.
- Integration and Expression: Once inside the host cell, the transgene integrates into the host genome, either randomly or at specific target sites using genome editing tools like CRISPR-Cas9. The integrated transgene is then expressed, leading to the production of the desired protein or trait in the transgenic organism.
Applications of Transgenesis
Transgenesis has a wide range of applications in various fields, including:
Agriculture
Transgenic crops have been developed to improve yield, nutritional value, and resistance to pests, diseases, and environmental stresses. Examples include Bt crops (expressing insecticidal proteins from Bacillus thuringiensis), herbicide-resistant crops, and biofortified crops with enhanced levels of essential nutrients.
Case Study: Golden Rice
Golden Rice is a transgenic rice variety engineered to produce beta-carotene, a precursor to vitamin A, in its grains. Developed to address vitamin A deficiency in developing countries, Golden Rice has the potential to improve the health of millions of people worldwide.
Animal Biotechnology
Transgenic animals are used as disease models for studying human disorders, producing biopharmaceuticals in their milk or eggs (pharming), and enhancing livestock traits such as growth rate, meat quality, and disease resistance. Notable examples include transgenic mice for cancer research and transgenic goats producing human antithrombin III in their milk.
Case Study: Enviro-Pig
The Enviropig is a transgenic pig that expresses a bacterial phytase gene, enabling it to digest phytate in its feed. This reduces the need for phosphorus supplementation and decreases the environmental impact of pig manure, addressing both nutritional and ecological challenges in pig farming.
Biomedical Research
Transgenic organisms, particularly mice, are invaluable tools for studying gene function, disease mechanisms, and drug development. By introducing human disease-causing genes into animals, researchers can create models that closely mimic human conditions, enabling the development of targeted therapies and treatments.
Case Study: Alzheimer's Disease Transgenic Mice
Transgenic mice expressing human genes associated with Alzheimer's disease, such as APP and PSEN1, have been developed to study the pathogenesis and progression of the disease. These models have contributed to the understanding of Alzheimer's and the development of potential therapeutic strategies.
Techniques for Creating Transgenic Organisms
Several techniques have been developed for creating transgenic organisms, each with its own advantages and limitations:
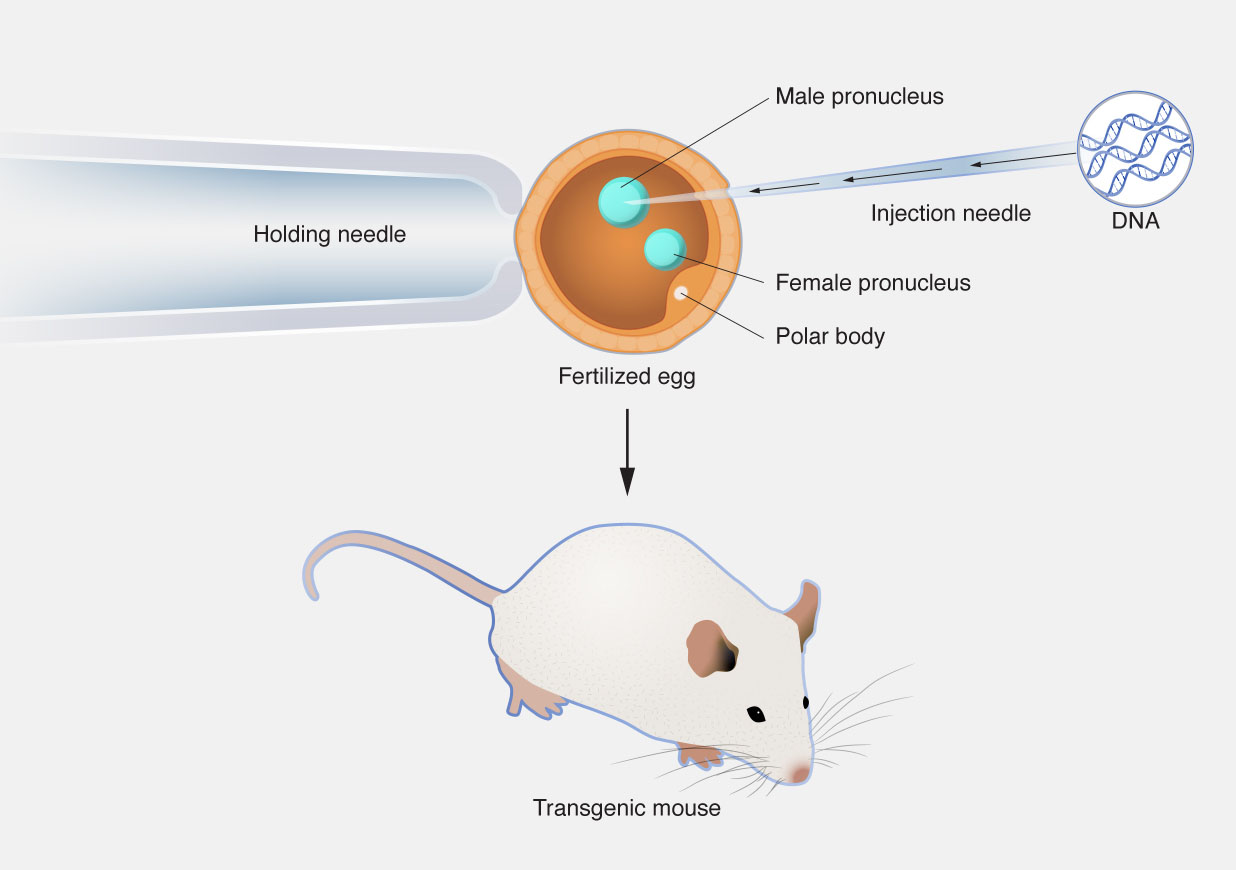
Pronuclear Microinjection
This technique involves the direct injection of the transgene into the pronucleus of a fertilized egg. It is widely used for generating transgenic mice and other mammals. While effective, it has limitations such as random integration and variable expression levels.
Embryonic Stem Cell-Mediated Gene Transfer
In this approach, the transgene is introduced into embryonic stem cells, which are then injected into early-stage embryos. The resulting chimeric animals are bred to establish stable transgenic lines. This method allows for targeted gene integration and the generation of knockout animals.
Viral Transduction
Retroviruses and lentiviruses can be engineered to carry the transgene and infect target cells, resulting in the integration of the transgene into the host genome. This technique is particularly useful for transgene delivery into hard-to-transfect cells and for in vivo gene therapy applications.
Genome Editing
The advent of genome editing tools, such as CRISPR-Cas9, has revolutionized the field of transgenesis. These tools enable precise and efficient modification of the host genome, allowing for targeted transgene integration, gene knockout, and gene correction. Genome editing has greatly expanded the possibilities for creating transgenic organisms with desired traits and characteristics.
Challenges in Transgenesis
Despite the significant advancements in transgenesis, several challenges persist:
- Low Efficiency: The success rate of producing transgenic organisms can be low, particularly when using traditional methods like pronuclear microinjection. This necessitates the use of a large number of embryos and extensive screening to identify successful transgenic individuals.
- Random Integration: Many transgenic techniques result in the random integration of the transgene into the host genome. This can lead to variable expression levels, unintended disruption of endogenous genes, and potential safety concerns.
- Mosaicism: Mosaicism occurs when not all cells in a transgenic organism carry the transgene, resulting in non-uniform expression. This can complicate the analysis and interpretation of transgenic phenotypes.
- Silencing and Stability: Transgenes may be subject to silencing over time due to epigenetic modifications or genomic instability. Ensuring stable and long-term expression of the transgene remains a challenge in some cases.
Researchers are actively working on developing strategies to overcome these challenges, such as using site-specific integration techniques, optimizing transgene design, and employing advanced screening methods to identify stable transgenic lines.
Ethical Considerations and Regulations
Transgenesis raises various ethical and safety concerns, particularly when it comes to the creation of transgenic animals and the potential ecological impact of transgenic organisms. Strict regulations and guidelines have been put in place to ensure the responsible development and use of transgenic organisms.
Ethical considerations include animal welfare, the potential for unintended consequences, and the societal implications of creating organisms with novel traits. Regulatory bodies, such as the FDA and USDA in the United States, oversee the development, testing, and commercialization of transgenic organisms to ensure their safety for human health and the environment.
Future Perspectives
Transgenesis continues to evolve with advancements in molecular biology, genome editing, and synthetic biology. Future research will focus on improving the precision and efficiency of transgene integration, developing novel delivery methods, and exploring the potential of transgenic organisms for addressing global challenges such as food security, disease prevention, and environmental sustainability.
The integration of transgenesis with other emerging technologies, such as gene drives and microbiome engineering, will open up new avenues for modifying organisms and ecosystems. However, these advancements also raise new ethical and regulatory challenges that will need to be addressed through public discourse and international collaboration.
Further Reading
Journal of Genetic Engineering and Biotechnology, A review of transgenic animal techniques and their applications
Brain Structure and Function, Animal transgenesis: an overview
Frontiers in Plant Science, Genetically engineered crops for sustainably enhanced food production systems
