Thymine (T): A Key Building Block of DNA
What is Thymine?
Thymine (T) is one of the four nucleobases found in DNA, alongside adenine (A), guanine (G), and cytosine (C). It is a pyrimidine derivative, meaning it has a single-ring structure. Thymine plays a crucial role in the structure and function of DNA, forming base pairs with adenine and contributing to the genetic code.
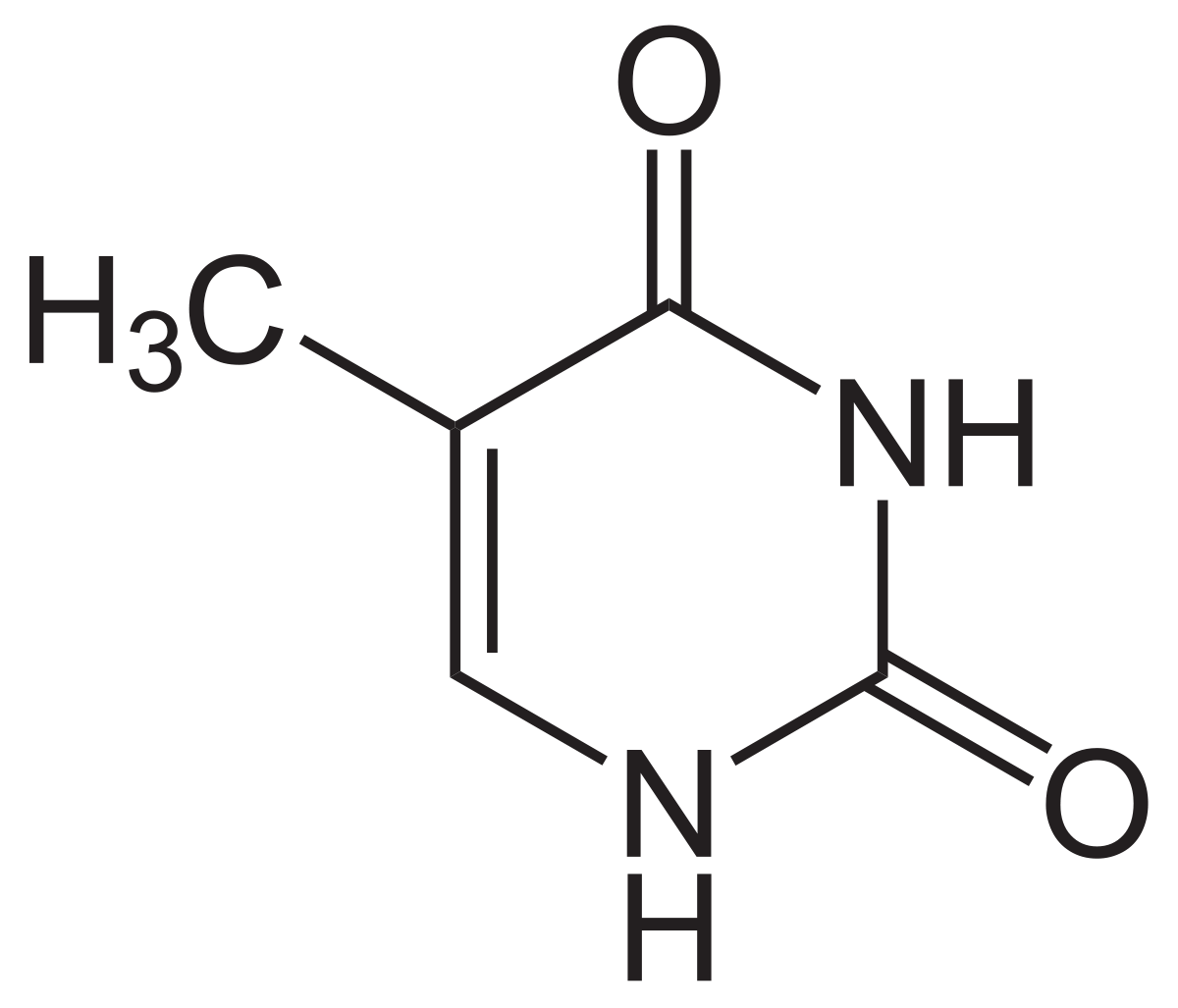
Chemical Structure and Properties
Thymine is a heterocyclic aromatic organic compound with the chemical formula C5H6N2O2. Its systematic name is 5-methyluracil, as it is a derivative of the RNA nucleobase uracil with an additional methyl group attached to the 5th carbon atom of the pyrimidine ring.
Key Properties of Thymine:
- Molecular Weight: 126.115 g/mol
- Melting Point: 316-317 °C
- Solubility: Slightly soluble in water, more soluble in hot water and organic solvents
- UV Absorption: Strong absorption at 260 nm
Thymine in DNA Structure
In the double helix structure of DNA, thymine forms a base pair with adenine through two hydrogen bonds. This specific pairing is known as complementary base pairing and is essential for the stability and replication of DNA. The thymine-adenine (T-A) base pair is held together by fewer hydrogen bonds compared to the guanine-cytosine (G-C) base pair, which has three hydrogen bonds.
Chargaff's Rule and DNA Composition
The complementary base pairing in DNA leads to a specific ratio of nucleobases, known as Chargaff's rule. According to this rule, the amount of adenine is equal to the amount of thymine, and the amount of guanine is equal to the amount of cytosine in a double-stranded DNA molecule. This 1:1 ratio of A:T and G:C is crucial for maintaining the structure and stability of DNA.
Thymine in the Genetic Code
The genetic code is determined by the sequence of nucleobases in DNA. Thymine, along with the other nucleobases, forms three-letter codons that specify amino acids during protein synthesis. Each codon consists of three consecutive nucleotides, and the order of these codons determines the sequence of amino acids in a protein.
Thymine-Containing Codons
Thymine is present in several codons that code for various amino acids, such as:
- TTT and TTC: Phenylalanine (Phe)
- TTA and TTG: Leucine (Leu)
- TCT, TCC, TCA, and TCG: Serine (Ser)
- TAT and TAC: Tyrosine (Tyr)
- TGT and TGC: Cysteine (Cys)
- TGA: Stop codon (termination of protein synthesis)
Thymine Derivatives and Modifications
Thymine can undergo various modifications that play important roles in DNA function and regulation. Some of these derivatives include:
5-Methylcytosine (5mC)
5-Methylcytosine is a modified nucleobase formed by the enzymatic addition of a methyl group to the 5th carbon of cytosine. This modification is involved in epigenetic regulation, gene silencing, and DNA methylation patterns. Although not directly related to thymine, 5mC is often referred to as the "fifth base" of DNA due to its significant biological role.
Thymine Dimers
Exposure to ultraviolet (UV) radiation can cause the formation of thymine dimers, where two adjacent thymine bases become covalently linked. This dimerization can lead to DNA damage and mutations if not repaired by cellular mechanisms. Thymine dimers are a common type of UV-induced DNA lesion and are associated with skin cancer risk.
Thymine in DNA Replication and Mutation
During DNA replication, the complementary base pairing of thymine with adenine ensures the accurate copying of genetic information. DNA polymerase enzymes use the existing DNA strand as a template and add the complementary nucleotides, including thymine, to the growing new strand.
Thymine-Related Mutations
Mutations involving thymine can occur during DNA replication or as a result of DNA damage. Some common thymine-related mutations include:
- Transition mutations: A change from thymine to cytosine (T → C) or from adenine to guanine (A → G) on the complementary strand.
- Transversion mutations: A change from thymine to adenine (T → A) or guanine (T → G).
- Thymine deletions or insertions: The loss or gain of one or more thymine bases in the DNA sequence.
These mutations can have various consequences, such as altering the amino acid sequence of proteins, introducing premature stop codons, or shifting the reading frame, potentially leading to altered gene function or genetic disorders.
Conclusion
Thymine is a fundamental building block of DNA, playing a critical role in the structure, stability, and genetic information storage of living organisms. Its complementary base pairing with adenine and its presence in the genetic code highlight its significance in molecular biology. Understanding the properties, functions, and modifications of thymine is essential for deciphering the complexities of the genetic code and advancing fields such as genetic engineering, molecular diagnostics, and personalized medicine.
Further Reading
Advances in Genome Biology, DNA: Structure and function
Essays in Biochemistry, Understanding biochemistry: structure and function of nucleic acids
Nature Reviews Chemistry, Methods for detection of cytosine and thymine modifications in DNA
