Single-Cell Sequencing Unveils Genetic Secrets of Individual Cells
What is Single-Cell Sequencing?
Single-cell sequencing is a revolutionary technology that allows researchers to analyze the genetic material of individual cells, providing unprecedented insights into cellular heterogeneity and function. Unlike traditional sequencing methods that analyze bulk samples containing millions of cells, single-cell sequencing enables the examination of the genome, transcriptome, or epigenome of single cells, revealing the unique genetic profiles and behaviors of each cell within a population.
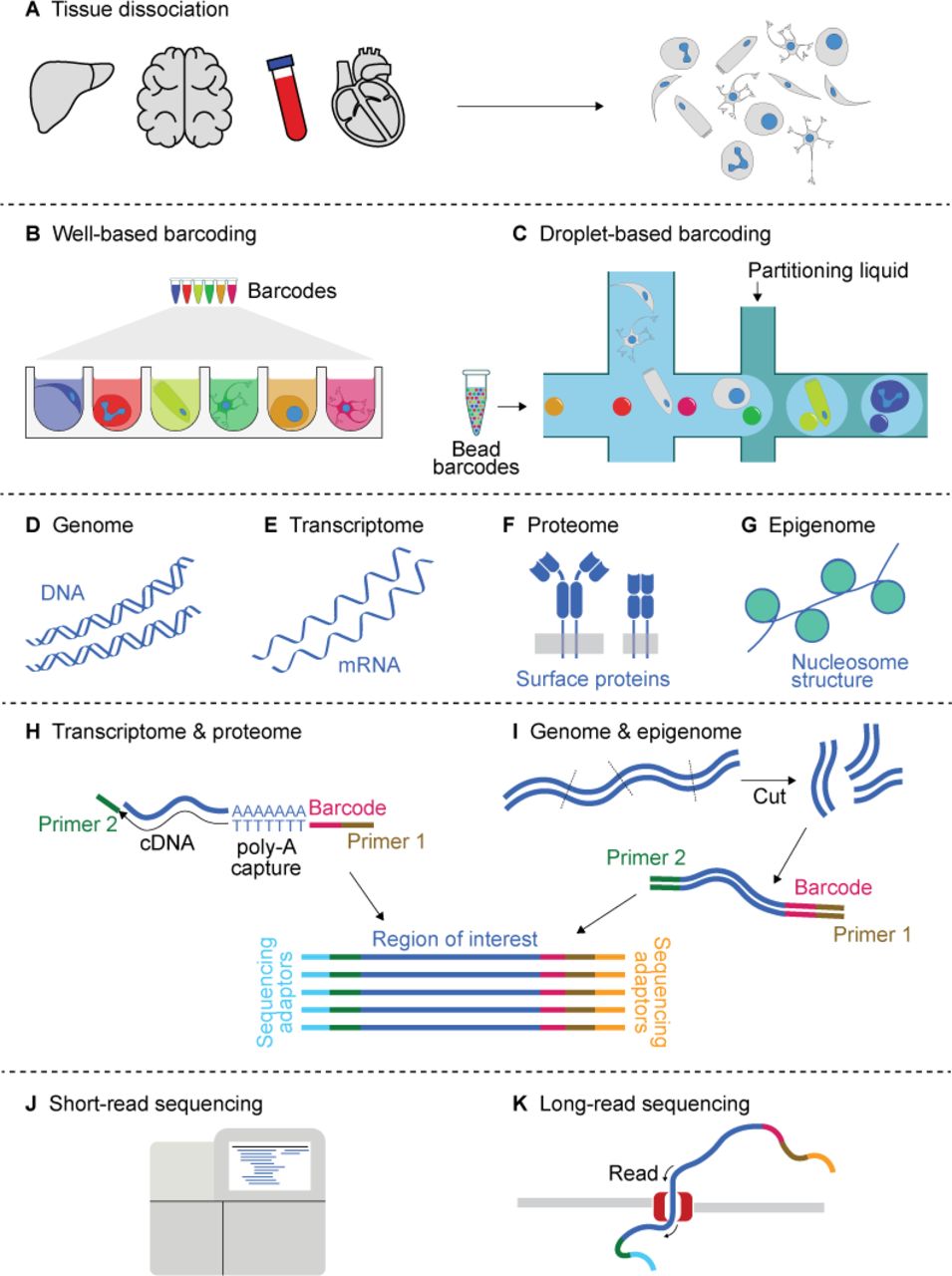
Key Steps in Single-Cell Sequencing
Single-cell sequencing involves several critical steps to isolate individual cells and analyze their genetic material:
- Cell Isolation: Individual cells are isolated from a heterogeneous sample using techniques such as microfluidics, laser capture microdissection, or fluorescence-activated cell sorting (FACS). This step ensures that each cell is physically separated from others to prevent cross-contamination.
- Cell Lysis and Nucleic Acid Extraction: The isolated cells are lysed to release their genetic material, which can be DNA for genome sequencing or RNA for transcriptome analysis. The nucleic acids are then extracted and purified for further processing.
- Amplification: Due to the minute amounts of genetic material in a single cell, amplification is necessary to generate sufficient copies for sequencing. Techniques such as multiple displacement amplification (MDA) or polymerase chain reaction (PCR) are commonly used to amplify the DNA or RNA.
- Library Preparation and Sequencing: The amplified genetic material is processed to create sequencing libraries, which are then sequenced using high-throughput sequencing platforms. The choice of sequencing technology depends on the specific application and desired coverage depth.
- Data Analysis: The sequencing data generated from individual cells is analyzed using bioinformatics tools and pipelines. This involves quality control, alignment to reference genomes, variant calling, and expression quantification. Specialized algorithms are employed to handle the unique challenges of single-cell data, such as high noise levels and technical variations.
Applications of Single-Cell Sequencing
Single-cell sequencing has revolutionized various fields of biology and medicine, enabling groundbreaking discoveries and applications:
Cellular Heterogeneity and Rare Cell Populations
Single-cell sequencing allows researchers to uncover the cellular heterogeneity within tissues and organs, revealing rare cell populations that may have critical roles in health and disease. By profiling individual cells, scientists can identify previously unrecognized cell types, understand their functions, and track their lineage relationships.
Developmental Biology and Stem Cell Research
Single-cell sequencing is a powerful tool for studying embryonic development and stem cell differentiation. By analyzing the transcriptomes of individual cells at different developmental stages, researchers can map the gene expression changes and regulatory networks that drive cell fate decisions and organ formation.
Cancer Biology and Precision Medicine
Single-cell sequencing has transformed our understanding of cancer biology by revealing the genetic heterogeneity within tumors. By sequencing individual cancer cells, researchers can identify rare tumor subpopulations, track clonal evolution, and uncover mechanisms of drug resistance. This knowledge paves the way for personalized cancer therapies and precision medicine approaches.
Immunology and Infectious Diseases
Single-cell sequencing enables the dissection of immune cell populations and their responses to pathogens or immunotherapies. By profiling individual immune cells, researchers can map the diversity of immune receptors, identify antigen-specific T cells, and unravel the mechanisms of immune regulation and dysfunction in diseases like autoimmunity and chronic infections.
Challenges and Future Perspectives
Despite the remarkable advances in single-cell sequencing, several challenges remain to be addressed. One major challenge is the limited amount of genetic material in a single cell, which can lead to amplification biases and noise in the sequencing data. Improving the efficiency and accuracy of amplification methods is an active area of research.
Another challenge is the computational analysis of large-scale single-cell sequencing datasets. Specialized algorithms and pipelines are needed to handle the high dimensionality and sparsity of single-cell data, as well as to integrate multiple omics layers for a comprehensive understanding of cellular states and dynamics.
Future developments in single-cell sequencing will focus on increasing the throughput and reducing the cost of the technology, enabling the analysis of millions of cells in a single experiment. The integration of single-cell sequencing with other technologies, such as spatial transcriptomics and proteomics, will provide a multi-dimensional view of cellular function and organization within tissues.
Moreover, the application of single-cell sequencing in clinical settings holds great promise for personalized medicine. By profiling the genetic and transcriptomic landscapes of individual patient cells, clinicians can potentially develop tailored diagnostic and therapeutic strategies based on the unique molecular signatures of each patient.
Further Reading
Nature Methods, The promise of single-cell genomics
Precision Clinical Medicine, Recent advances in single-cell sequencing technologies
Journal of Human Genetics, Single-cell genomics to understand disease pathogenesis
