Ribosome: The Protein Synthesis Powerhouse of the Cell
What is a Ribosome?
Ribosomes are complex molecular machines found in all living cells that are responsible for synthesizing proteins from amino acids based on the genetic instructions encoded in messenger RNA (mRNA). They are essential for the growth, development, and survival of organisms across all domains of life.
The discovery of ribosomes dates back to the 1950s, when George Palade first observed dense particles in the cytoplasm of cells using electron microscopy. These particles were later named "ribosomes" by Richard B. Roberts in 1958. Subsequent studies by James Watson, Francis Crick, and others revealed the central role of ribosomes in protein synthesis, laying the foundation for our current understanding of these fascinating molecular machines.
Structure and Composition of Ribosomes
Ribosomes are composed of two main subunits: a small subunit and a large subunit. In eukaryotes, the small subunit is called the 40S subunit, while the large subunit is called the 60S subunit. In prokaryotes, the small subunit is known as the 30S subunit, and the large subunit is the 50S subunit.
Each subunit consists of ribosomal RNA (rRNA) and ribosomal proteins. The rRNA forms the structural and functional core of the ribosome, while the proteins provide additional support and help in the assembly and stability of the ribosome.
Small Subunit
The small subunit of the ribosome is responsible for binding and decoding the mRNA. It contains the decoding center, where the codons of the mRNA are read and matched with the corresponding anticodon of the transfer RNA (tRNA) molecules carrying the appropriate amino acids.
Large Subunit
The large subunit of the ribosome contains the peptidyl transferase center (PTC), which catalyzes the formation of peptide bonds between the amino acids, leading to the elongation of the protein chain. The large subunit also provides the exit tunnel through which the nascent polypeptide chain emerges from the ribosome.
Molecular Interactions within the Ribosome
During protein synthesis, the ribosome undergoes a series of dynamic conformational changes and molecular interactions that enable the accurate decoding of the genetic information and the efficient production of proteins.
The small subunit of the ribosome contains the mRNA binding channel, where the mRNA is threaded and positioned for decoding. The rRNA of the small subunit, particularly the 16S rRNA in prokaryotes and the 18S rRNA in eukaryotes, plays a crucial role in the decoding process. The rRNA forms specific interactions with the mRNA and the tRNA molecules, ensuring the fidelity of codon-anticodon base pairing.
In the large subunit, the peptidyl transferase center is formed primarily by the 23S rRNA in prokaryotes and the 28S rRNA in eukaryotes. The rRNA catalyzes the peptide bond formation between the amino acids, without the need for additional protein enzymes. The rRNA also contributes to the formation of the exit tunnel, which provides a protected environment for the nascent polypeptide chain to fold and emerge from the ribosome.
The ribosomal proteins, although not directly involved in catalysis, play essential roles in the assembly, stability, and regulation of the ribosome. They help maintain the correct folding and positioning of the rRNA, facilitate the binding and release of translation factors, and contribute to the overall structural integrity of the ribosome.
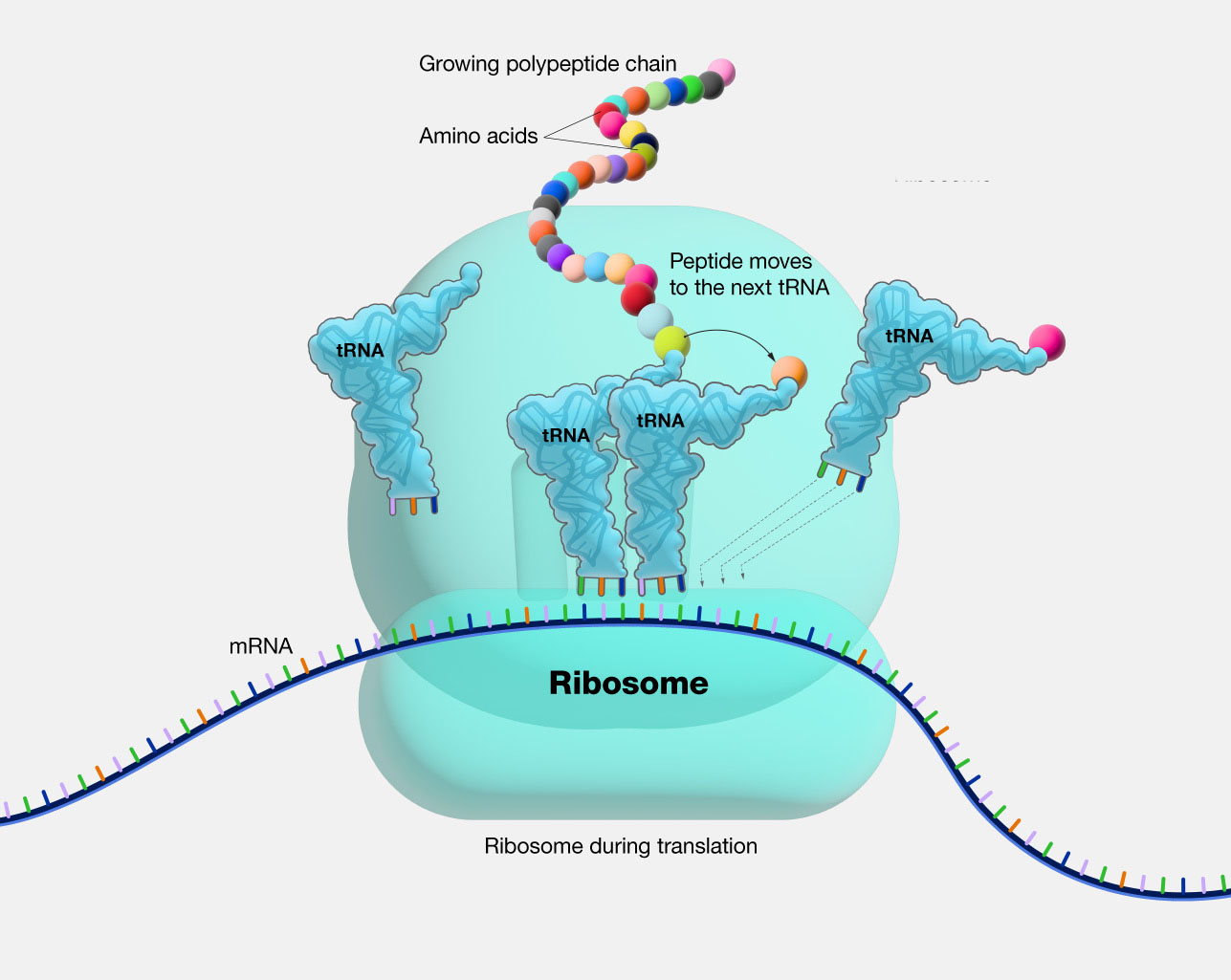
The Process of Protein Synthesis
Ribosomes play a central role in the process of protein synthesis, which involves three main stages: initiation, elongation, and termination.
Initiation
During initiation, the small subunit of the ribosome binds to the mRNA at the start codon (usually AUG) with the help of initiation factors and the initiator tRNA. The large subunit then joins the small subunit to form the complete ribosome, ready to begin protein synthesis.
Elongation
Elongation involves the repetitive addition of amino acids to the growing polypeptide chain. The ribosome moves along the mRNA, reading the codons one by one. For each codon, the corresponding tRNA molecule carrying the appropriate amino acid binds to the ribosome. The peptidyl transferase center then catalyzes the formation of a peptide bond between the new amino acid and the existing polypeptide chain. The ribosome then translocates to the next codon, and the process continues.
Termination
Termination occurs when the ribosome encounters a stop codon (UAA, UAG, or UGA) on the mRNA. Release factors bind to the ribosome and cause the release of the completed polypeptide chain. The ribosomal subunits then dissociate from the mRNA and are recycled for the next round of protein synthesis.
Ribosome Biogenesis and Regulation
The biogenesis of ribosomes is a complex and highly regulated process that involves the coordinated expression and assembly of rRNA and ribosomal proteins. In eukaryotes, ribosome biogenesis occurs primarily in the nucleolus, a specialized compartment within the nucleus.
The regulation of ribosome biogenesis and function is crucial for maintaining cellular homeostasis and responding to various physiological and environmental cues. Factors such as nutrient availability, growth factors, and stress signals can influence ribosome biogenesis and modulate protein synthesis rates.
Ribosomopathies and Therapeutic Targeting
Ribosomopathies are a group of disorders caused by mutations in genes encoding ribosomal proteins or factors involved in ribosome biogenesis. These disorders often manifest as developmental abnormalities, bone marrow failure, and increased risk of cancer. Examples of ribosomopathies include Diamond-Blackfan anemia, Shwachman-Diamond syndrome, and dyskeratosis congenita.
Ribosomes have also emerged as potential targets for antimicrobial and anticancer therapies. Many antibiotics, such as tetracyclines and aminoglycosides, inhibit bacterial protein synthesis by binding to and disrupting the function of bacterial ribosomes. In cancer, ribosome biogenesis and protein synthesis are often upregulated to support the increased growth and proliferation of tumor cells. Targeting ribosomes and their associated pathways could provide new avenues for cancer treatment.
Future Perspectives
Despite significant advances in our understanding of ribosome structure and function, many aspects of ribosome biology remain to be explored. The development of high-resolution cryo-electron microscopy and other structural biology techniques has revolutionized the study of ribosomes, providing unprecedented insights into their dynamic conformations and interactions with various ligands.
Future research will focus on elucidating the mechanisms of ribosome assembly, quality control, and regulation in different cellular contexts. The role of ribosomes in disease pathogenesis and their potential as therapeutic targets will continue to be an area of active investigation. Additionally, the study of ribosomes from diverse organisms, including extremophiles and ancient lineages, may shed light on the evolution and adaptability of these essential molecular machines.
Further Reading
Nature Reviews Molecular Cell Biology, A Structural Understanding of the Dynamic Ribosome Machine
Protein | Science, The Ribosome in Action: Tuning Translation Efficiency and Protein Folding
Nature Structural & Molecular Biology, Eukaryotic ribosome assembly, transport and quality control
