Protein: The Building Blocks of Life
What are Proteins?
Proteins are essential macromolecules that play a crucial role in virtually all biological processes. They are the building blocks of life, performing a wide range of functions within organisms, from catalyzing metabolic reactions to providing structural support and enabling cell signaling. Proteins are composed of one or more chains of amino acids, which are linked together by peptide bonds and folded into specific three-dimensional structures.
Amino Acids: The Building Blocks of Proteins
Proteins are built from a set of 20 standard amino acids, each with a specific side chain that gives it unique chemical properties. Amino acids are joined together by peptide bonds to form polypeptide chains. The sequence of amino acids in a protein, known as its primary structure, is determined by the genetic code. This sequence ultimately dictates the protein's three-dimensional structure and function.
Protein Synthesis
Proteins are synthesized through a process called translation, which occurs in the ribosomes of cells. The genetic information stored in DNA is first transcribed into Messenger RNA (mRNA), which then serves as a template for protein synthesis. Transfer RNA (tRNA) molecules, each carrying a specific amino acid, recognize the codons on the mRNA and bring the corresponding amino acids to the ribosome. The amino acids are then linked together to form the polypeptide chain, which folds into its native three-dimensional structure.
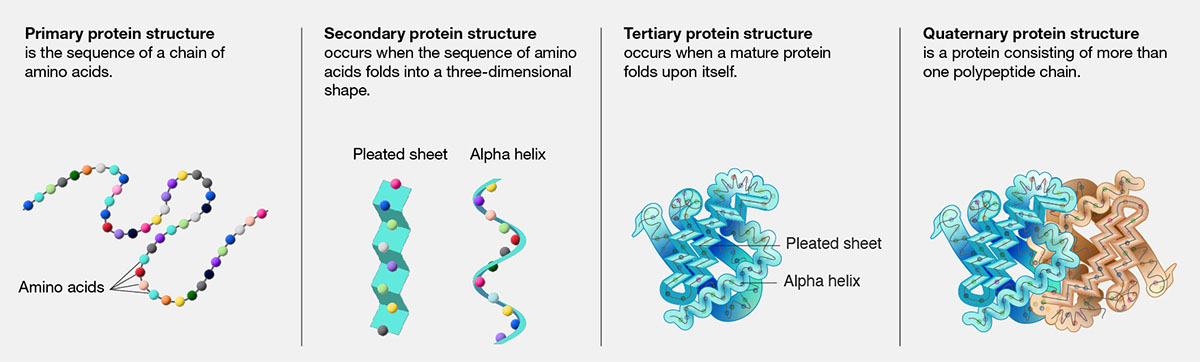
Protein Structure
Proteins have four levels of structure that contribute to their unique shapes and functions:
- Primary Structure: The linear sequence of amino acids in a polypeptide chain.
- Secondary Structure: Local folding patterns within the polypeptide chain, such as alpha helices and beta sheets, stabilized by hydrogen bonds.
- Tertiary Structure: The overall three-dimensional shape of a single polypeptide chain, stabilized by interactions between the side chains of amino acids, such as hydrophobic interactions, hydrogen bonds, and disulfide bridges.
- Quaternary Structure: The assembly of multiple polypeptide chains (subunits) into a multi-subunit complex.
Protein Functions
Proteins perform a wide variety of functions in living organisms, including:
- Enzymes: Catalyze biochemical reactions, increasing their rate and specificity.
- Structural Proteins: Provide mechanical support and maintain cell shape, e.g., collagen, elastin, and keratin.
- Transport Proteins: Move molecules across cell membranes or throughout the body, e.g., hemoglobin and ion channels.
- Signaling Proteins: Transmit signals between cells or within a cell, e.g., hormones, receptors, and G proteins.
- Antibodies: Recognize and bind to foreign substances (antigens) as part of the immune response.
- Regulatory Proteins: Control gene expression and cell activities, e.g., transcription factors and repressors.
Protein-Protein Interactions
Proteins often interact with other proteins to form complexes or networks that carry out specific biological functions. These interactions can be transient or stable and are mediated by specific domains or motifs within the proteins. Understanding protein-protein interactions is crucial for deciphering cellular processes and developing targeted therapies for diseases.
Protein Misfolding and Diseases
Proper protein folding is essential for maintaining their biological functions. However, proteins can sometimes misfold, leading to the formation of aggregates or fibrils that are associated with various diseases, such as Alzheimer's, Parkinson's, and Huntington's. Studying the mechanisms of protein misfolding and developing strategies to prevent or reverse it is an active area of research in biotechnology and medicine.
Protein Engineering and Applications
Advances in biotechnology have enabled the engineering of proteins with novel or improved functions. Techniques such as site-directed mutagenesis, directed evolution, and rational design allow researchers to modify the amino acid sequence, stability, and activity of proteins. Engineered proteins have a wide range of applications, including:
- Development of new enzymes for industrial processes
- Design of novel therapeutics, such as antibody-drug conjugates
- Creation of biosensors and diagnostic tools
- Improvement of crop traits through genetic modification
Studying Proteins
Various techniques are used to study proteins, their structures, and their functions, including:
- X-ray Crystallography: Determines the three-dimensional structure of proteins by analyzing the diffraction patterns of X-rays passed through a crystallized protein sample.
- Nuclear Magnetic Resonance (NMR) Spectroscopy: Provides information about the structure and dynamics of proteins in solution by exploiting the magnetic properties of atomic nuclei.
- Cryo-Electron Microscopy (Cryo-EM): Allows the visualization of large protein complexes and macromolecular assemblies by imaging frozen samples with electron beams.
- Mass Spectrometry: Identifies and quantifies proteins in complex mixtures by measuring the mass-to-charge ratios of ionized protein fragments.
These techniques, along with bioinformatics tools and computational modeling, have greatly advanced our understanding of proteins and their roles in biological systems.
Further Reading
Protein Engineering, Protein Engineering: Past, Present, and Future
Essays in Biochemistry, Uncovering protein function: from classification to complexes
World Journal of Biological Chemistry, Proteomics: Concepts and applications in human medicine