Monoclonal Antibodies: Precision-Engineered Therapeutic Agents
What are Monoclonal Antibodies?
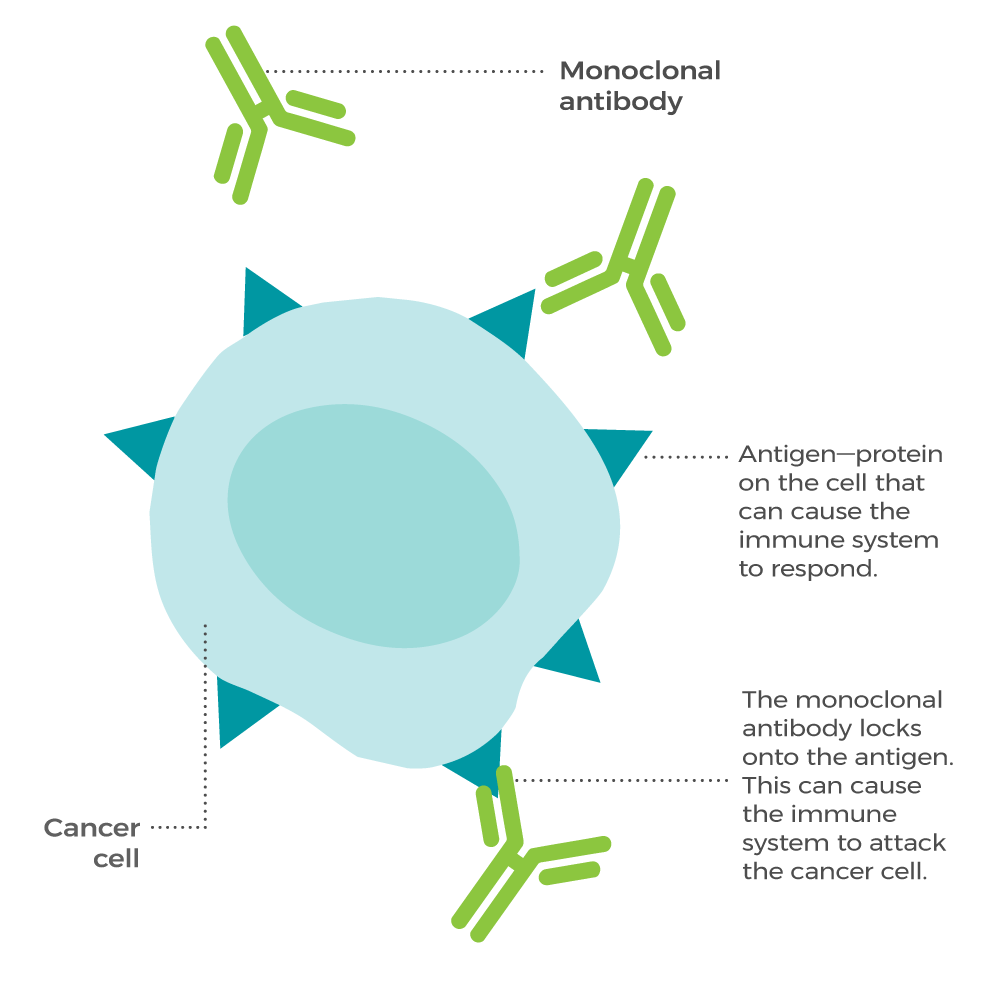
Monoclonal antibodies (mAbs) are highly specific, laboratory-produced antibodies designed to target a single specific epitope or binding site on an antigen. Unlike polyclonal antibodies, which are a mixture of antibodies produced by different B cell clones, monoclonal antibodies are derived from a single B cell clone and recognize only one epitope on an antigen. This unique specificity makes monoclonal antibodies powerful tools in research, diagnostics, and therapeutics.
Production of Monoclonal Antibodies
The production of monoclonal antibodies involves a multi-step process called hybridoma technology, which was first developed by Georges Köhler and César Milstein in 1975. The key steps in this process include:
- Immunization: An animal, typically a mouse, is immunized with the desired antigen to stimulate the production of antibodies against that specific antigen.
- B Cell Isolation: B cells are isolated from the spleen of the immunized animal. These B cells produce antibodies specific to the antigen used for immunization.
- Fusion: The isolated B cells are fused with immortal myeloma cells to create hybridoma cells. Myeloma cells are cancerous B cells that can grow indefinitely in culture.
- Selection: The hybridoma cells are cultured in a selective medium that allows only the fused cells to survive. Unfused B cells have a limited lifespan, while unfused myeloma cells die in the selective medium.
- Screening: The surviving hybridoma cells are screened for the production of the desired antibody using techniques such as enzyme-linked immunosorbent assay (ELISA) or flow cytometry.
- Cloning: The selected hybridoma cell that produces the desired antibody is cloned to establish a stable cell line that secretes monoclonal antibodies with uniform specificity and affinity.
- Production: The established hybridoma cell line is cultured in large-scale bioreactors to produce large quantities of monoclonal antibodies, which are then purified for various applications.
Types of Monoclonal Antibodies
There are several types of monoclonal antibodies based on their origin and structure:
- Murine Antibodies: These are derived from mouse B cells and are 100% mouse protein. They have a higher potential for immunogenicity in humans.
- Chimeric Antibodies: These antibodies have a mouse variable region and a human constant region. They are about 65% human and have reduced immunogenicity compared to murine antibodies.
- Humanized Antibodies: These antibodies have a human framework with only the complementarity-determining regions (CDRs) derived from a mouse. They are about 95% human and have significantly reduced immunogenicity.
- Fully Human Antibodies: These antibodies are derived from human B cells or produced using transgenic mice with human immunoglobulin genes. They have the lowest potential for immunogenicity.
Mechanisms of Action
Therapeutic monoclonal antibodies can elicit various mechanisms of action to target and eliminate diseased cells or modulate cellular processes. Some of the key mechanisms include:
- Neutralization: Antibodies can bind to specific antigens, such as viruses or toxins, and neutralize their activity, preventing them from infecting or damaging cells.
- Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC): Antibodies can bind to target cells and recruit immune effector cells, such as natural killer cells, which then release cytotoxic granules to kill the target cells.
- Complement-Dependent Cytotoxicity (CDC): Antibodies can activate the complement system, leading to the formation of membrane attack complexes that lyse the target cells.
- Signal Transduction Inhibition: Antibodies can bind to cell surface receptors and block the binding of ligands, inhibiting downstream signaling pathways that promote cell growth or survival.
- Antibody-Drug Conjugates (ADCs): Antibodies can be conjugated to cytotoxic drugs, enabling targeted delivery of the drug to specific cells while minimizing systemic toxicity.
Applications of Monoclonal Antibodies
Monoclonal antibodies have found extensive applications in various fields due to their high specificity and affinity. Some of the major applications include:
Therapeutics
Monoclonal antibodies have revolutionized the field of targeted therapy for various diseases, including cancer, autoimmune disorders, and infectious diseases. Therapeutic monoclonal antibodies can specifically bind to disease-associated antigens and elicit various mechanisms of action, such as blocking signaling pathways, inducing apoptosis, or activating the immune system. Examples of FDA-approved therapeutic monoclonal antibodies include trastuzumab (Herceptin) for breast cancer, adalimumab (Humira) for rheumatoid arthritis, and rituximab (Rituxan) for non-Hodgkin's lymphoma.
Diagnostics
Monoclonal antibodies are widely used in diagnostic assays to detect the presence of specific antigens in biological samples. They are employed in various formats, such as ELISA, immunohistochemistry, flow cytometry, and lateral flow assays (e.g., pregnancy tests). Monoclonal antibodies enable highly sensitive and specific detection of biomarkers, pathogens, and other molecules of interest, facilitating early diagnosis and monitoring of diseases.
Research
Monoclonal antibodies are invaluable tools in basic and applied research. They are used to study protein function, localization, and interactions, as well as to purify proteins using immunoaffinity chromatography. Monoclonal antibodies also serve as reagents for the development of new assays, validation of potential drug targets, and investigation of cellular processes and signaling pathways.
Regulatory Considerations and Biosimilars
The development and approval of monoclonal antibodies are subject to stringent regulatory requirements. The regulatory pathway involves preclinical studies, clinical trials, and a thorough review process by regulatory agencies such as the FDA (Food and Drug Administration) in the United States or the EMA (European Medicines Agency) in Europe. The approval process ensures the safety, efficacy, and quality of monoclonal antibody therapeutics.
With the expiration of patents for many originator monoclonal antibodies, there has been a growing interest in the development of biosimilars. Biosimilars are highly similar versions of approved monoclonal antibodies that have no clinically meaningful differences in terms of safety, purity, and potency. The introduction of biosimilars aims to increase competition, improve patient access, and reduce healthcare costs while maintaining the same therapeutic benefits as the reference products.
Challenges and Future Perspectives
While monoclonal antibodies have proven to be highly effective in various applications, there are still challenges to overcome. One of the main challenges is the potential immunogenicity of monoclonal antibodies, especially when derived from non-human sources. This can lead to the development of anti-drug antibodies, which may reduce the efficacy of the treatment and cause adverse reactions. To mitigate this issue, researchers have developed humanized and fully human monoclonal antibodies using techniques such as phage display and transgenic mice.
Another challenge is the high cost associated with the development and production of monoclonal antibodies. The complex manufacturing process, stringent quality control requirements, and lengthy clinical trials contribute to the substantial expense of monoclonal antibody therapies. Efforts are being made to improve production efficiency, reduce costs, and explore alternative production platforms, such as plant-based systems and microbial hosts.
The future of monoclonal antibodies is promising, with ongoing research focusing on the development of novel antibody formats, such as bispecific antibodies, antibody-drug conjugates, and nanobodies. These innovations aim to enhance the therapeutic efficacy, reduce side effects, and expand the range of targetable antigens. Additionally, the integration of monoclonal antibodies with other technologies, such as gene editing and cell therapy, holds great potential for personalized medicine and targeted therapies for various diseases.
Further Reading
Antibody Therapeutics, The global landscape of approved antibody therapies
Journal of Biomedical Science, Development of therapeutic antibodies for the treatment of diseases
Current Opinion in Pharmacology, Monoclonal antibody therapeutics: history and future
