Genome Editing: Precise Manipulation of DNA for Biotechnology Advancement
What is Genome Editing?
Genome editing is a powerful biotechnology tool that allows scientists to make precise changes to an organism's DNA. It involves the use of specialized enzymes, known as engineered nucleases or "molecular scissors," to cut DNA at specific locations and then modify, insert, or delete genetic material at that site. This technology has revolutionized the field of genetic engineering, enabling researchers to study gene function, develop novel therapies, and improve agricultural crops.
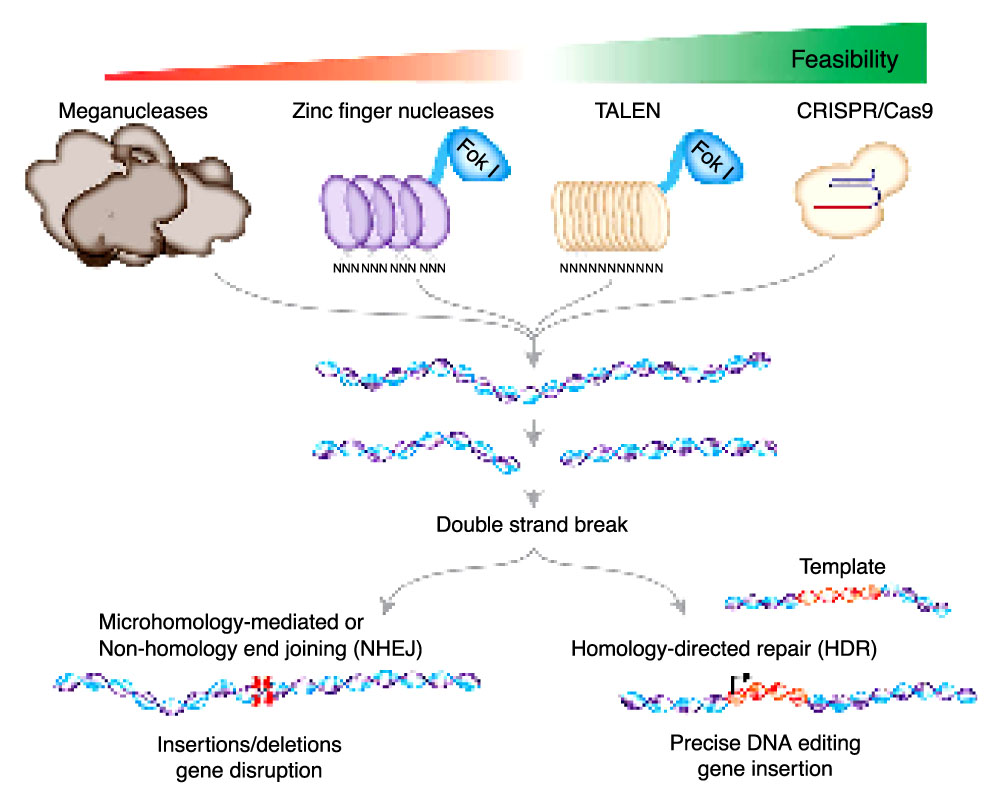
Key Genome Editing Technologies
Several genome editing technologies have been developed, each with its own advantages and limitations:
CRISPR-Cas Systems
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) and its associated Cas (CRISPR-associated) proteins, particularly Cas9, have emerged as the most popular and versatile genome editing tools. CRISPR-Cas systems are adapted from a naturally occurring bacterial defense mechanism against viral infections. The Cas9 endonuclease is guided by a single guide RNA (sgRNA) to a specific DNA sequence, where it creates a double-strand break. This break can then be repaired through either non-homologous end joining (NHEJ) or homology-directed repair (HDR), leading to the desired genetic modification.
Zinc Finger Nucleases (ZFNs)
Zinc Finger Nucleases (ZFNs) are engineered proteins that consist of a DNA-binding zinc finger protein fused to a DNA-cleaving nuclease domain. The zinc finger protein can be designed to recognize specific DNA sequences, enabling targeted genome editing. ZFNs work in pairs, with each pair member recognizing a specific half-site. When both ZFNs bind to their respective half-sites, the nuclease domains dimerize and create a double-strand break in the DNA.
TALENs
Transcription Activator-Like Effector Nucleases (TALENs) are similar to ZFNs in their mode of action. They are composed of a DNA-binding TALE protein derived from Xanthomonas bacteria, fused to a DNA-cleaving nuclease domain. The TALE protein consists of highly conserved repeat domains, each recognizing a single DNA base pair. By assembling multiple TALE repeats, researchers can design TALENs to target specific DNA sequences for genome editing.
Emerging Technologies
In addition to the well-established genome editing tools, novel technologies such as base editing and prime editing are being developed. Base editing allows for the direct conversion of one DNA base pair to another without creating double-strand breaks, while prime editing combines a Cas9 nickase with a reverse transcriptase to enable more precise and versatile genetic modifications.
Gene Editing vs. Genome Editing
While gene editing and genome editing are often used interchangeably, they have subtle but important differences that distinguish them:
Gene Editing
Definition: Gene editing involves the precise alteration of specific genes within an organism's DNA. This can include adding, removing, or modifying segments of a gene to achieve a desired change.
Scope: The focus is typically on individual genes or small segments of DNA, making the modifications highly targeted.
Applications: Common applications of gene editing include correcting genetic defects, introducing new traits, or studying gene function. Techniques like CRISPR-Cas9 are often used for these precise edits.
Genome Editing
Definition: Genome editing is a broader term that encompasses the editing of any part of an organism's genome, which includes all of its genetic material. This can involve more extensive changes, including multiple genes or regulatory regions.
Scope: Genome editing can involve larger-scale modifications, affecting large sections of the genome or multiple genes simultaneously. It is not limited to single-gene changes.
Applications: While it shares applications with gene editing, such as correcting genetic defects and introducing new traits, genome editing can also include more complex modifications like altering regulatory elements, creating large deletions or insertions, or even editing entire chromosomes.
Applications of Genome Editing
Genome editing has a wide range of applications in basic research, biotechnology, and medicine:
Basic Research
Genome editing tools have greatly facilitated the study of gene function and the creation of animal models for human diseases. By introducing precise genetic modifications, researchers can investigate the roles of specific genes in development, physiology, and disease pathogenesis. This knowledge is crucial for understanding the molecular basis of biological processes and identifying potential targets for therapeutic intervention.
Agriculture
Genome editing is being applied to improve agricultural crops by introducing desirable traits such as increased yield, enhanced nutritional value, and resistance to pests and environmental stresses. By making precise genetic modifications, researchers can accelerate the development of improved crop varieties without the need for extensive breeding programs. This technology has the potential to contribute to global food security and sustainable agriculture.
Medicine
Genome editing holds great promise for the development of novel therapies for genetic diseases, cancer, and infectious diseases. By correcting disease-causing mutations or introducing protective genetic modifications, genome editing could potentially cure or prevent many debilitating conditions. Researchers are exploring the use of genome editing in somatic cells for the treatment of diseases such as sickle cell anemia, beta-thalassemia, cystic fibrosis, and Duchenne muscular dystrophy. In recent clinical trials, CRISPR-Cas9 has shown encouraging results in treating sickle cell disease and beta-thalassemia by editing hematopoietic stem cells. Additionally, genome editing is being investigated as a tool for enhancing the efficacy and safety of cell-based therapies, such as CAR T-cell therapy for cancer treatment.
Ethical Considerations and Regulations
The rapid advancement of genome editing technologies has raised important ethical and societal questions. The potential for off-target effects, where unintended genetic modifications occur, is a significant concern. Researchers are working to improve the specificity and efficiency of genome editing tools to minimize these risks.
The use of genome editing in human embryos for reproductive purposes is highly controversial and is currently prohibited in many countries. There are concerns about the safety, efficacy, and long-term consequences of germline genome editing, as well as the potential for the creation of "designer babies" with enhanced traits. The case of He Jiankui, a Chinese scientist who claimed to have created the world's first gene-edited babies using CRISPR-Cas9, sparked global outrage and highlighted the need for strict regulations and oversight in this field.
Regulatory frameworks for genome editing are evolving to keep pace with the rapid developments in the field. International guidelines, such as those provided by the World Health Organization (WHO) and the International Commission on the Clinical Use of Human Germline Genome Editing, aim to ensure responsible and ethical use of this technology.
Future Perspectives
Genome editing is a rapidly evolving field with immense potential to transform biology, agriculture, and medicine. Ongoing research aims to improve the precision, efficiency, and delivery of genome editing tools. The development of novel genome editing technologies, such as base editing and prime editing, is expanding the repertoire of genetic modifications that can be made.
As genome editing becomes more accessible and cost-effective, its applications are expected to expand. However, it is crucial to address the ethical, legal, and societal implications of this technology to ensure its responsible and equitable use for the benefit of humanity.
Further Reading
Annual Review of Pharmacology and Toxicology, Genome Editing: A New Approach to Human Therapeutics
