Enzymes: Biological Catalysts Essential for Life
What are Enzymes?
Enzymes are biological catalysts that speed up chemical reactions in living organisms. They are essential for life, as they play a crucial role in various biological processes, such as digestion, metabolism, and cell regulation. Enzymes are primarily proteins, although some RNA molecules called ribozymes also exhibit enzymatic activity.
Key Characteristics of Enzymes
Enzymes possess several unique characteristics that make them highly efficient and specific catalysts:
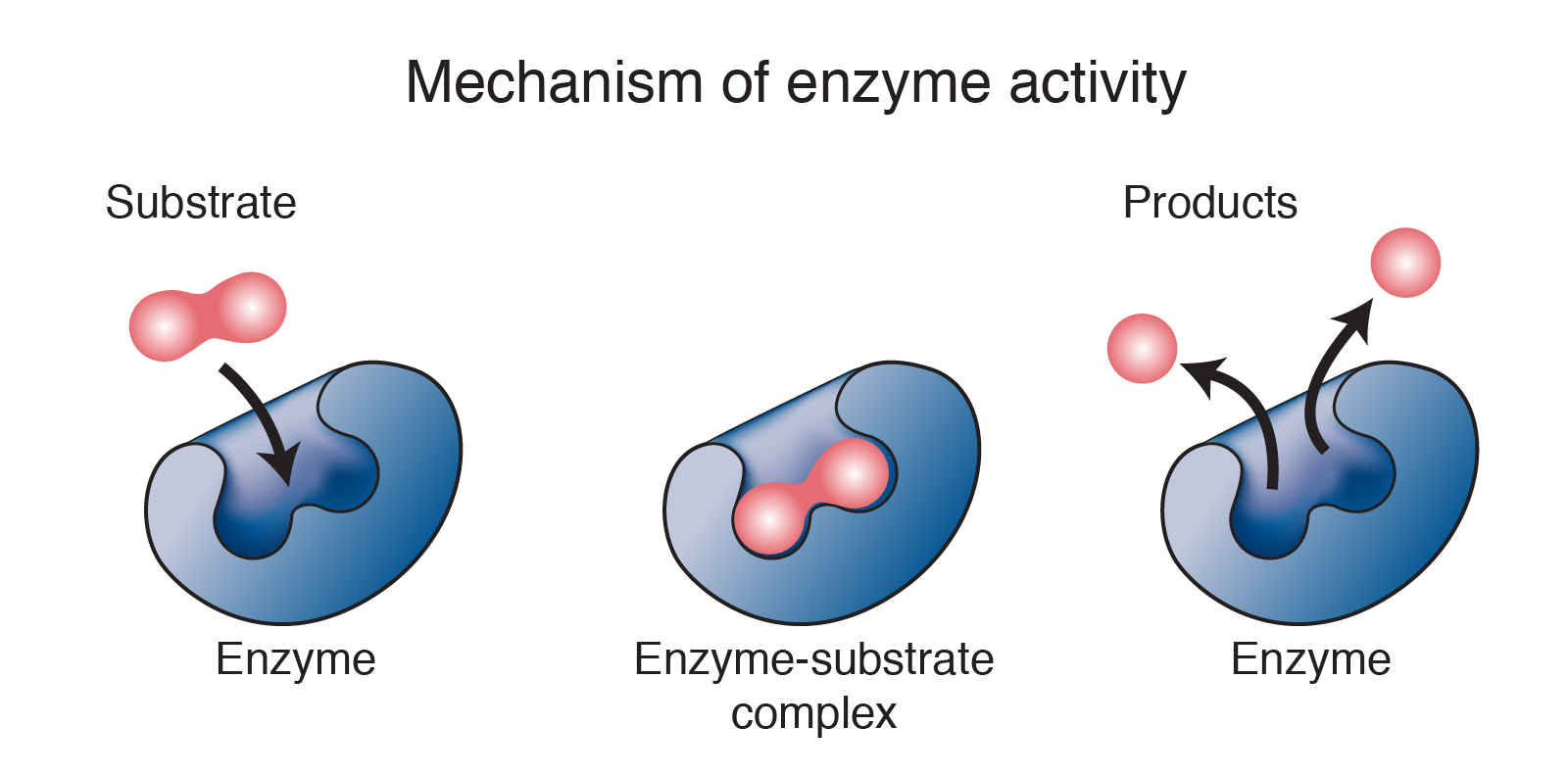
Substrate Specificity
Enzymes are highly specific to their substrates, the molecules upon which they act. The active site of an enzyme has a unique three-dimensional structure that allows it to bind to a specific substrate, much like a lock and key. This specificity ensures that enzymes catalyze only the desired reactions, preventing unwanted side reactions.
Catalytic Efficiency
Enzymes are remarkably efficient catalysts, often increasing reaction rates by several orders of magnitude compared to uncatalyzed reactions. They achieve this by lowering the activation energy required for the reaction to occur, stabilizing transition states, and providing an optimal environment for the reaction.
Regulation and Control
Enzyme activity is tightly regulated in living organisms to maintain homeostasis and respond to changing conditions. Enzymes can be regulated by various means, such as allosteric regulation (binding of effector molecules), covalent modification (e.g., phosphorylation), and changes in pH or temperature.
Classification of Enzymes
Enzymes are classified into six main categories based on the type of reaction they catalyze:
- Oxidoreductases: Catalyze oxidation-reduction reactions, involving the transfer of electrons between molecules.
- Transferases: Transfer functional groups (e.g., methyl, phosphate) from one molecule to another.
- Hydrolases: Catalyze hydrolysis reactions, breaking bonds using water.
- Lyases: Catalyze non-hydrolytic addition or removal of groups from substrates, often forming double bonds.
- Isomerases: Catalyze the rearrangement of molecules, resulting in isomeric forms.
- Ligases: Catalyze the joining of two molecules, typically coupled with the hydrolysis of ATP.
Enzyme Kinetics and Michaelis-Menten Equation
Enzyme kinetics is the study of the rates of enzyme-catalyzed reactions. The Michaelis-Menten equation is a fundamental concept in enzyme kinetics, describing the relationship between the reaction rate and substrate concentration:
v = (Vmax * [S]) / (KM + [S])
Where:
- v is the reaction rate
- Vmax is the maximum reaction rate
- [S] is the substrate concentration
- KM is the Michaelis constant, which represents the substrate concentration at which the reaction rate is half of Vmax
The Michaelis-Menten equation helps researchers understand the behavior of enzymes under different conditions and can be used to determine important kinetic parameters, such as Vmax and KM.
Enzyme Inhibition
Enzyme inhibition is the process by which the activity of an enzyme is reduced or blocked by a molecule called an inhibitor. There are three main types of enzyme inhibition:
- Competitive Inhibition: The inhibitor competes with the substrate for the active site of the enzyme, reducing the amount of enzyme available to bind to the substrate.
- Non-competitive Inhibition: The inhibitor binds to a site other than the active site, causing a conformational change that reduces the enzyme's activity.
- Uncompetitive Inhibition: The inhibitor binds only to the enzyme-substrate complex, preventing the formation of the product.
Enzyme inhibition is a critical aspect of drug design, as many drugs act as enzyme inhibitors to treat various diseases, such as hypertension, cancer, and viral infections.
Applications of Enzymes
Enzymes have a wide range of applications in various fields, including:
- Medicine: Enzymes are used as therapeutic agents (e.g., thrombolytic enzymes for treating blood clots) and in diagnostic tests (e.g., glucose oxidase for measuring blood sugar).
- Biotechnology: Enzymes are used in the production of biofuels, detergents, and food products (e.g., cheese, beer).
- Agriculture: Enzymes are used to improve crop yields, enhance animal feed digestion, and control pests.
- Environmental Science: Enzymes are used in bioremediation to degrade pollutants and clean up contaminated sites.
Future Perspectives
As our understanding of enzymes continues to grow, researchers are exploring new ways to harness their power for various applications. Some key areas of focus include:
- Enzyme engineering: Designing and modifying enzymes to improve their stability, specificity, and catalytic efficiency.
- Immobilized enzymes: Attaching enzymes to solid supports to enhance their reusability and stability in industrial processes.
- Synergistic enzyme systems: Combining multiple enzymes to catalyze complex, multi-step reactions.
- Therapeutic enzymes: Developing enzyme-based therapies for treating genetic disorders, cancer, and other diseases.
By advancing our knowledge of enzymes and their applications, we can unlock new possibilities in medicine, biotechnology, and environmental science, leading to a more sustainable and healthier future.
Further Reading
Essays in Biochemistry, Enzymes: principles and biotechnological applications
Journal of Environmental Management, A comprehensive review on the potential of microbial enzymes in multipollutant bioremediation: Mechanisms, challenges, and future prospects
