Amino Acids: Building Blocks of Life
What are Amino Acids?
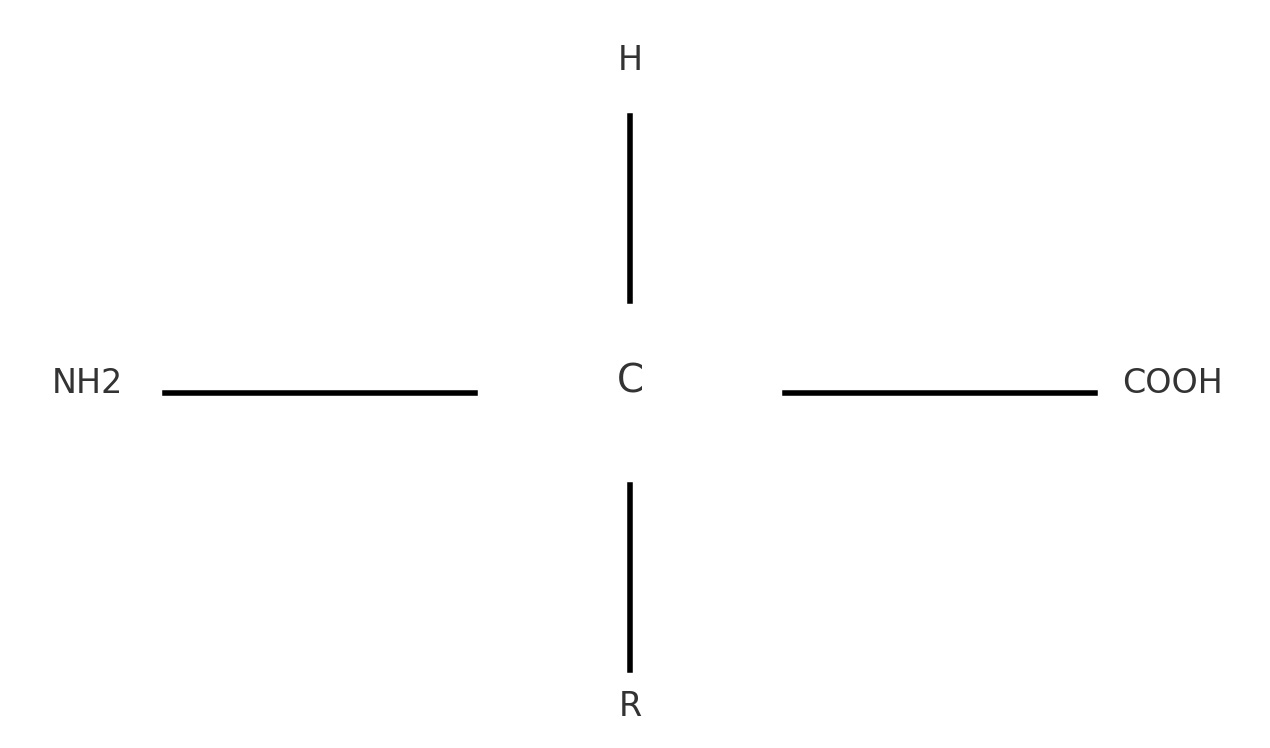
Amino acids are organic compounds that serve as the fundamental building blocks of proteins. They play a crucial role in the structure, function, and regulation of the body's tissues and organs. Amino acids are characterized by their central carbon atom, known as the α-carbon, which is bonded to an amino group (-NH2), a carboxyl group (-COOH), a hydrogen atom, and a variable side chain (R-group) that determines the unique properties of each amino acid.
Types of Amino Acids
There are 20 standard amino acids that are commonly found in proteins. These amino acids can be classified based on various criteria, such as their essentiality, polarity, and side chain properties.
Essential and Non-Essential Amino Acids
Amino acids can be categorized as essential or non-essential based on the body's ability to synthesize them:
- Essential Amino Acids: These amino acids cannot be synthesized by the body and must be obtained through dietary sources. The nine essential amino acids are histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine.
- Non-Essential Amino Acids: These amino acids can be synthesized by the body and do not need to be obtained through diet. The eleven non-essential amino acids are alanine, arginine, asparagine, aspartic acid, cysteine, glutamic acid, glutamine, glycine, proline, serine, and tyrosine.
Polarity and Side Chain Properties
Amino acids can also be classified based on the polarity and properties of their side chains:
- Polar Amino Acids: These amino acids have side chains that are hydrophilic and can form hydrogen bonds with water. Examples include serine, threonine, cysteine, asparagine, and glutamine.
- Non-Polar Amino Acids: These amino acids have side chains that are hydrophobic and do not interact well with water. Examples include alanine, valine, leucine, isoleucine, proline, phenylalanine, tryptophan, and methionine.
- Charged Amino Acids: These amino acids have side chains that carry a net positive or negative charge at physiological pH. Positively charged amino acids include lysine, arginine, and histidine, while negatively charged amino acids include aspartic acid and glutamic acid.
Peptide Bond Formation
Amino acids can be joined together through peptide bonds to form peptides and proteins. A peptide bond is a covalent bond that forms between the carboxyl group of one amino acid and the amino group of another, with the release of a water molecule. This process, known as condensation or dehydration synthesis, results in the formation of a linear chain of amino acids.
The sequence of amino acids in a protein, known as its primary structure, determines its unique three-dimensional shape and function. Proteins can further fold and assemble into complex structures, such as α-helices and β-sheets, which are stabilized by various interactions, including hydrogen bonds, disulfide bridges, and hydrophobic interactions.
Functions of Amino Acids
Amino acids and the proteins they form have diverse functions in the body, including:
- Structural Components: Proteins such as collagen, elastin, and keratin provide structural support and strength to tissues like skin, bones, and hair.
- Enzymes: Many proteins function as enzymes, catalyzing biochemical reactions and regulating metabolic processes.
- Hormones and Signaling Molecules: Some amino acids serve as precursors for the synthesis of hormones and neurotransmitters, such as thyroid hormones, dopamine, and serotonin.
- Antibodies: Proteins called antibodies are crucial components of the immune system, helping to defend the body against foreign invaders.
- Transport and Storage: Proteins like hemoglobin and myoglobin transport and store oxygen in the blood and muscles, respectively.
Amino Acid Metabolism
Amino acid metabolism involves the synthesis, breakdown, and interconversion of amino acids in the body. Excess amino acids that are not used for protein synthesis can be catabolized to produce energy or converted into other molecules, such as glucose or fatty acids. Disorders of amino acid metabolism can lead to various health conditions, such as phenylketonuria, maple syrup urine disease, and homocystinuria.
Amino Acids in Biotechnology and Medicine
Amino acids have numerous applications in biotechnology and medicine. They are used in the production of pharmaceuticals, dietary supplements, and food additives. Amino acids can also be modified or incorporated into peptides and proteins to create novel biomaterials with specific properties and functions.
In medicine, amino acid-based therapies are being explored for the treatment of various conditions, such as cancer, metabolic disorders, and neurodegenerative diseases. For example, the amino acid L-DOPA is used to treat Parkinson's disease, while L-glutamine supplementation is being investigated for its potential to reduce the side effects of cancer chemotherapy.
Further Reading
Signal Transduction and Targeted Therapy, Amino acid metabolism in health and disease
Frontiers in Chemistry, Application of Amino Acids in the Structural Modification of Natural Products: A Review
