| Nov 10, 2023 | |
Biomimetic nanoparticles offer promise for enhanced detection of circulating tumor cells |
|
| (Nanowerk Spotlight) Detecting cancer at an early stage is crucial for effective treatment and improving patient outcomes. A key challenge is finding ways to detect minute numbers of circulating tumor cells (CTCs) in blood samples. CTCs have broken away from the original tumor site and entered the bloodstream, acting as an early warning sign that cancer may be spreading or metastasizing to other areas of the body. | |
| Being able to accurately detect CTCs from a standard blood draw could allow oncologists to diagnose cancers sooner and select targeted therapies, as well as monitor treatment effectiveness. | |
| However, CTCs occur at extremely low frequencies amidst billions of normal blood cells. This needle-in-a-haystack problem makes it difficult to positively identify and isolate CTCs for further analysis. Current CTC detection platforms struggle to differentiate CTCs from the vast numbers of white blood cells also present. As a result, isolated samples still contain high levels of white blood cell "contaminants", hampering downstream genomic or proteomic profiling of the scarce CTCs themselves. | |
| Seeking to improve CTC isolation purity, scientists at Nankai University in China have now developed cell membrane-coated magnetic nanoparticles (MNs) that leverage special abilities of two different cell types. As reported in the journal Advanced Functional Materials ("Genetically Engineered Cell Membrane-Coated Magnetic Nanoparticles for High-Performance Isolation of Circulating Tumor Cells"), the team's biomimetic nanoparticles can both specifically target CTCs and evade non-specific binding to white blood cells. | |
 |
|
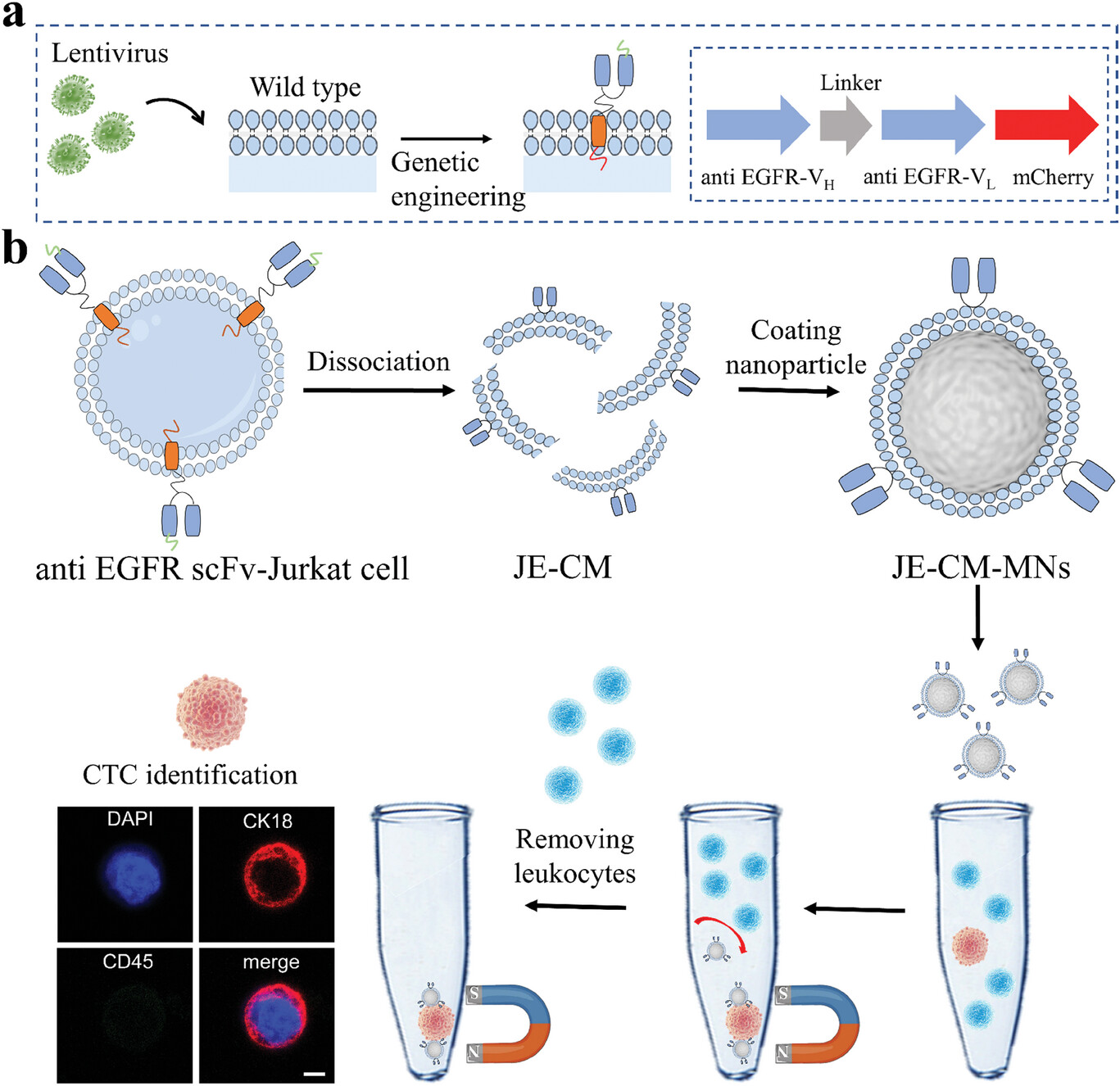
| Construction of the genetically engineered cell membrane-based platform for isolation of CTCs. a) Schematic illustration of the construction of genetically engineered cell. Anti-EGFR scFv-Jurkat cells were obtained by Jurkat cell with lentivirus treatment. b) The fabrication of JE-CM-MNs and the assay process of CTC enrichment and identification. Scale bar, 5 µm. | |
| The researchers cloak magnetic nanoparticle cores with extracted cell membranes from Jurkat immune cells. This leukocyte membrane camouflage allows the particles to evade detection by white blood cells, similar to how viruses use membrane coating to invade host cells unseen. Tests showed the disguised nanoparticles had far fewer interactions with white blood cells compared to naked nanoparticles in blood samples. | |
| “The cell membrane plays an essential role in many physiological and pathological processes,” said Dr. Lailiang Ou, a senior author of the paper. “Recent studies have illustrated that the leukocyte membrane coating can reduce the interaction between particles and leukocytes, which may be considered an ideal camouflage coating toward homologous cells.” | |
| At the same time, the team displays on the nanoparticle surfaces single-chain variable antibody fragments (scFv) that target epidermal growth factor receptor, a protein commonly overexpressed in cancer cells. This chimeric antibody membrane technology was achieved by genetically modifying Jurkat cells to stably express the tumor-hunting scFv on their outer membrane. Extruding the engineered cell membranes onto magnetic nanoparticles transferred this selective CTC-capturing capability to the nanoparticles. | |
| By modifying the cell membrane and then coating the nanoparticles with it, the team was able to equip the nanoparticles with both white blood cell evasion abilities and specific CTC targeting abilities. | |
| To test the performance of the engineered nanoparticles, the researchers added (or spiked) known quantities of cancer cells into blood samples from healthy donors. This created artificial blood samples containing a controlled number of cancer cells to mimic real clinical blood draws. | |
| The engineered nanoparticles were then added to these spiked blood samples. Compared to commercial CTC-targeting nanoparticles tested on the same spiked samples, the new nanoparticles captured significantly more cancer cells - 80% versus 52% for the commercial nanoparticles. Interestingly, the cell membrane coating concurrently reduced white blood cell background to just 7% of all isolated cells, a nearly 10-fold purity improvement. | |
| “Our platform exhibited excellent specificity and sensitive detection in clinical blood samples and enabled practical cancer diagnosis of patients with a detection rate of 100%,” explained Dr. Xinglu Huang, co-author of the study. | |
| The ability to non-invasively detect CTCs with high yield and purity could prove a boon for early cancer screening and treatment monitoring. Before clinical adoption, further validation is needed with larger patient sample sizes. There is also opportunity to expand the approach by targeting diverse membrane proteins overexpressed across cancer types. This innovative biomimetic nanoparticle platform represents a promising new tactic in the fight against metastatic cancer. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
