Showing Spotlights 33 - 40 of 150 in category All (newest first):
 Researchers a new strategy for designing Pickering emulsion based on halloysite nanotubes and exploring chitosan and pectin as thickener additives for the development of cleaning and preservation applications for cultural heritage. This novel and facile strategy to prepare hydrogel containing oil-in-water stable emulsion offers an alternative route to prepare formulations with high performance and based on sustainable materials for the controlled cleaning and preservation of stone-based artworks.
Researchers a new strategy for designing Pickering emulsion based on halloysite nanotubes and exploring chitosan and pectin as thickener additives for the development of cleaning and preservation applications for cultural heritage. This novel and facile strategy to prepare hydrogel containing oil-in-water stable emulsion offers an alternative route to prepare formulations with high performance and based on sustainable materials for the controlled cleaning and preservation of stone-based artworks.
May 8th, 2019
 To perform a risk assessment of nanomaterials in the environment, information on the exposure, i.e. the amounts that are present in the environment, is essential. In contrast to many other known pollutants, the concentrations of nanomaterials in environmental systems cannot be measured directly. In this situation, exposure modelling is a solution to estimate the environmental exposure with synthetic nanomaterials. In order to predict the amount of a nanomaterial in a certain environmental compartment (environmental exposure) present in a certain area, two basic types of models are required - material flow analysis and environmental behaviour modelling.
To perform a risk assessment of nanomaterials in the environment, information on the exposure, i.e. the amounts that are present in the environment, is essential. In contrast to many other known pollutants, the concentrations of nanomaterials in environmental systems cannot be measured directly. In this situation, exposure modelling is a solution to estimate the environmental exposure with synthetic nanomaterials. In order to predict the amount of a nanomaterial in a certain environmental compartment (environmental exposure) present in a certain area, two basic types of models are required - material flow analysis and environmental behaviour modelling.
Apr 23rd, 2019
 In new work, researchers explore inexpensive, biodegradable and daily-waste eggshell membrane as a novel bio-piezoelectric material for harvesting green energy. The uniqueness of our work lies in the novelty of directly utilizing natural eggshell membranes as efficient piezoelectric material. This simple, innovative approach could provide huge benefits for research in future energy science, especially with regard to in vivo biomedical applications.
In new work, researchers explore inexpensive, biodegradable and daily-waste eggshell membrane as a novel bio-piezoelectric material for harvesting green energy. The uniqueness of our work lies in the novelty of directly utilizing natural eggshell membranes as efficient piezoelectric material. This simple, innovative approach could provide huge benefits for research in future energy science, especially with regard to in vivo biomedical applications.
Jun 12th, 2018
 Widespread use of synthetic agrochemicals in crop protection has led to serious concerns of environmental contamination and increased resistance in plant-based pathogenic microbes. In an effort to develop bio-based and non-synthetic alternatives, nanobiotechnology researchers are looking to plants that possess natural antimicrobial properties. In new work, researchers show that nanoscale thymol's antibacterial and antifungal properties not only prevent plant disease but that it also enhances plant growth.
Widespread use of synthetic agrochemicals in crop protection has led to serious concerns of environmental contamination and increased resistance in plant-based pathogenic microbes. In an effort to develop bio-based and non-synthetic alternatives, nanobiotechnology researchers are looking to plants that possess natural antimicrobial properties. In new work, researchers show that nanoscale thymol's antibacterial and antifungal properties not only prevent plant disease but that it also enhances plant growth.
May 4th, 2018
 One of the main challenges for energy efficient technologies is to lower their cost by making cheap energy-efficient materials and devices by preferably using green manufacturing technologies. For example, commercial infrared-blocking windows, both passive and active, are simply too expensive (most of these IR-blocking windows contain an expensive silver coating) and they are not used in the majority of our homes. One approach to this problem involves creating passive infrared-blocking glasses using plasmonic nanocrystals. Researchers have demonstrated that nanocrystals of relatively inexpensive plasmonic materials show an overall good performance as IR-blocking elements.
One of the main challenges for energy efficient technologies is to lower their cost by making cheap energy-efficient materials and devices by preferably using green manufacturing technologies. For example, commercial infrared-blocking windows, both passive and active, are simply too expensive (most of these IR-blocking windows contain an expensive silver coating) and they are not used in the majority of our homes. One approach to this problem involves creating passive infrared-blocking glasses using plasmonic nanocrystals. Researchers have demonstrated that nanocrystals of relatively inexpensive plasmonic materials show an overall good performance as IR-blocking elements.
Apr 27th, 2018
 Carbon nanomaterials, including graphene-based materials, are widely gaining popularity in practical applications of nanomanufacturing. As a result, it becomes more and more likely that the unwanted introduction of such materials into the environment may occur. In particular, aqueous habitats might be severely affected by any accidental carbon nanomaterials exposure. Researching these potential environmental toxicity effects, scientists have found that kaolin, a cheap and abundant clay, can act as a powerful antidote to remediate the toxic effects of graphene oxide.
Carbon nanomaterials, including graphene-based materials, are widely gaining popularity in practical applications of nanomanufacturing. As a result, it becomes more and more likely that the unwanted introduction of such materials into the environment may occur. In particular, aqueous habitats might be severely affected by any accidental carbon nanomaterials exposure. Researching these potential environmental toxicity effects, scientists have found that kaolin, a cheap and abundant clay, can act as a powerful antidote to remediate the toxic effects of graphene oxide.
Apr 25th, 2018
 Turning atmospheric carbon dioxide (CO2) into valuable products seems like a great idea to help remove this greenhouse gas to mitigate climate change. Using a process of molten carbonate electrolytic transformation of CO2 to carbon nanotubes, researchers have now demonstrated 'carbon nanotube wool'. These are the first carbon nanotubes that can be directly woven into a cloth as they are of macroscopic length (over 1mm) and are cheap to produce. The sole reactant to produce the carbon nanotube wools is carbon dioxide. This transforms CO2 from a pollutant into a useful, valuable resource.
Turning atmospheric carbon dioxide (CO2) into valuable products seems like a great idea to help remove this greenhouse gas to mitigate climate change. Using a process of molten carbonate electrolytic transformation of CO2 to carbon nanotubes, researchers have now demonstrated 'carbon nanotube wool'. These are the first carbon nanotubes that can be directly woven into a cloth as they are of macroscopic length (over 1mm) and are cheap to produce. The sole reactant to produce the carbon nanotube wools is carbon dioxide. This transforms CO2 from a pollutant into a useful, valuable resource.
Jul 18th, 2017
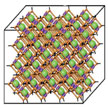 Metal-organic frameworks (MOFs) are well-ordered, lattice-like crystals. The nodes of the lattices are metals, which are connected by organic molecules. The most impressive features of MOFs are their extremely high surface area, high porosity, and tunable pore sizes, which are remarkable advantages over other porous materials (e.g., zeolites and carbons). With their special structure and large surface area, MOFs open up new opportunities for alternative systems for gas and energy storage (e.g. carbon dioxide and hydrogen storage), in catalysis, chemical sensing, as nanoreactors, and in drug delivery, making them hugely interesting for both university research and industry.
Metal-organic frameworks (MOFs) are well-ordered, lattice-like crystals. The nodes of the lattices are metals, which are connected by organic molecules. The most impressive features of MOFs are their extremely high surface area, high porosity, and tunable pore sizes, which are remarkable advantages over other porous materials (e.g., zeolites and carbons). With their special structure and large surface area, MOFs open up new opportunities for alternative systems for gas and energy storage (e.g. carbon dioxide and hydrogen storage), in catalysis, chemical sensing, as nanoreactors, and in drug delivery, making them hugely interesting for both university research and industry.
Jun 15th, 2017
 Researchers a new strategy for designing Pickering emulsion based on halloysite nanotubes and exploring chitosan and pectin as thickener additives for the development of cleaning and preservation applications for cultural heritage. This novel and facile strategy to prepare hydrogel containing oil-in-water stable emulsion offers an alternative route to prepare formulations with high performance and based on sustainable materials for the controlled cleaning and preservation of stone-based artworks.
Researchers a new strategy for designing Pickering emulsion based on halloysite nanotubes and exploring chitosan and pectin as thickener additives for the development of cleaning and preservation applications for cultural heritage. This novel and facile strategy to prepare hydrogel containing oil-in-water stable emulsion offers an alternative route to prepare formulations with high performance and based on sustainable materials for the controlled cleaning and preservation of stone-based artworks.
 Subscribe to our Nanotechnology Spotlight feed
Subscribe to our Nanotechnology Spotlight feed





